Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice
- PMID: 30209035
- PMCID: PMC6148195
- DOI: 10.1128/CMR.00057-17
Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice
Abstract
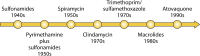
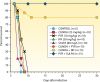
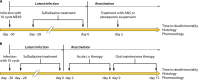
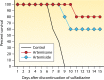
Primary Toxoplasma gondii infection is usually subclinical, but cervical lymphadenopathy or ocular disease can be present in some patients. Active infection is characterized by tachyzoites, while tissue cysts characterize latent disease. Infection in the fetus and in immunocompromised patients can cause devastating disease. The combination of pyrimethamine and sulfadiazine (pyr-sulf), targeting the active stage of the infection, is the current gold standard for treating toxoplasmosis, but failure rates remain significant. Although other regimens are available, including pyrimethamine in combination with clindamycin, atovaquone, clarithromycin, or azithromycin or monotherapy with trimethoprim-sulfamethoxazole (TMP-SMX) or atovaquone, none have been found to be superior to pyr-sulf, and no regimen is active against the latent stage of the infection. Furthermore, the efficacy of these regimens against ocular disease remains uncertain. In multiple studies, systematic screening for Toxoplasma infection during gestation, followed by treatment with spiramycin for acute maternal infections and with pyr-sulf for those with established fetal infection, has been shown to be effective at preventing vertical transmission and minimizing the severity of congenital toxoplasmosis (CT). Despite significant progress in treating human disease, there is a strong impetus to develop novel therapeutics for both the acute and latent forms of the infection. Here we present an overview of toxoplasmosis treatment in humans and in animal models. Additional research is needed to identify novel drugs by use of innovative high-throughput screening technologies and to improve experimental models to reflect human disease. Such advances will pave the way for lead candidates to be tested in thoroughly designed clinical trials in defined patient populations.
Keywords: T. gondii; Toxoplasma gondii; animal models; clindamycin; in vitro; in vivo; pyrimethamine; sulfadiazine; therapy; treatment.
Copyright © 2018 American Society for Microbiology.
Figures






References
-
- Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W, Valery N, Peneau C, Daigre JL, Aznar C, Cottrelle B, Terzan L, Darde ML, Carme B. 2007. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clin Infect Dis 45:e88–e95. doi: 10.1086/521246. - DOI - PubMed
-
- Khan AA, Araujo FG. 1996. Recent developments in the search for therapeutic interventions against Toxoplasma gondii infection, p 65–77. In Recent research developments in antimicrobial agents and chemotherapy, vol 1 Research Signpost, Thiruvananthapuram, Kerala, India.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

