Claudin-4 reconstituted in unilamellar vesicles is sufficient to form tight interfaces that partition membrane proteins
- PMID: 30209136
- PMCID: PMC6398474
- DOI: 10.1242/jcs.221556
Claudin-4 reconstituted in unilamellar vesicles is sufficient to form tight interfaces that partition membrane proteins
Abstract
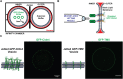
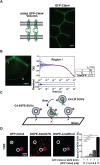
Tight junctions have been hypothesized to act as molecular fences in the plasma membrane of epithelial cells, helping to form differentiated apical and basolateral domains. While this fence function is believed to arise from the interaction of four-pass transmembrane claudins, the complexity of tight junctions has made direct evidence of their role as a putative diffusion barrier difficult to obtain. Here, we address this challenge by reconstituting claudin-4 into giant unilamellar vesicles using microfluidic jetting. We find that reconstituted claudin-4 alone can form adhesive membrane interfaces without the accessory proteins that are present in vivo By controlling the molecular composition of the inner and outer leaflets of jetted vesicle membranes, we show that claudin-4-mediated interfaces can drive partitioning of extracellular membrane proteins with ectodomains as small as 5 nm but not of inner or outer leaflet lipids. Our findings indicate that homotypic interactions of claudins and their small size can contribute to the polarization of epithelial cells.
Keywords: Cell adhesion; Epithelial cells; Polarity; Reconstitution; Tight junction; Transmembrane protein.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Diamond J. M. (1977). Twenty-first Bowditch lecture. The epithelial junction: bridge, gate, and fence. Physiologist 20, 10-18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

