Influenza A Virus Infection Causes Chronic Lung Disease Linked to Sites of Active Viral RNA Remnants
- PMID: 30209189
- PMCID: PMC6179922
- DOI: 10.4049/jimmunol.1800671
Influenza A Virus Infection Causes Chronic Lung Disease Linked to Sites of Active Viral RNA Remnants
Abstract
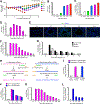
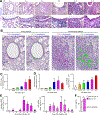
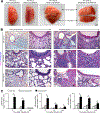
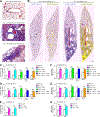
Clinical and experimental observations suggest that chronic lung disease is linked to respiratory viral infection. However, the long-term aspect of this relationship is not yet defined using a virus that replicates at properly high levels in humans and a corresponding animal model. In this study, we show that influenza A virus infection achieves 1 × 106-fold increases in viral load in the lung and dose-dependent severity of acute illness in mice. Moreover, these events are followed by persistence of negative- and positive-strand viral RNA remnants for 15 wk and chronic lung disease for at least 26 wk postinfection. The disease is manifested by focal areas of bronchiolization and mucus production that contain increased levels of viral RNA remnants along with mucin Muc5ac and Il13 mRNA compared with uninvolved areas of the lung. Excess mucus production and associated airway hyperreactivity (but not fibrosis or emphysema) are partially attenuated with loss of IL-13 production or signaling (using mice with IL-13 or STAT6 deficiency). These deficiencies cause reciprocal increases in l17a mRNA and neutrophils in the lung; however, none of these disease endpoints are changed with IL-13/IL-17a compared with IL-13 deficiency or STAT6/IL-17a compared with STAT6 deficiency. The results establish the capacity of a potent human respiratory virus to produce chronic lung disease focally at sites of active viral RNA remnants, likely reflecting locations of viral replication that reprogram the region. Viral dose dependency of disease also implicates high-level viral replication and severity of acute infection as determinants of chronic lung diseases such as asthma and COPD with IL-13-dependent and IL-13/IL-17-independent mechanisms.
Copyright © 2018 by The American Association of Immunologists, Inc.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest.
Figures





References
-
- Sigurs N, Gustafsson PM, Bjarnason R, Lundbeg F, Schmidt S, Sigurbergsson F, and Kjellman B. 2005. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med 171: 137–141. - PubMed
-
- Guilbert TW, Singh AM, Danov Z, Evans MD, Jackson DJ, Burton R, Roberg KA, Anderson EL, Pappas TE, Gangnon R, Gern JE, and Lemanske RF Jr. 2011. Decreased lung function after preschool wheezing rhinovirus illness in children at risk to develop asthma. J. Allergy Clin. Immunol 128: 532–538. - PMC - PubMed
-
- Kan-o K, Ramirez R, MacDonald MI, Rolph M, Rudd PA, Spann KM, Mahalingam S, Bardin PG, and Thomas BJ. 2017. Human metapneumovirus infection in chronic obstructive pulmonary disease: impact of glucocorticosteroids and inteferon. J. Inf. Dis 215: 1536–1545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

