LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis
- PMID: 30209220
- PMCID: PMC6166828
- DOI: 10.1073/pnas.1812196115
LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis
Abstract
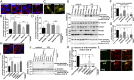
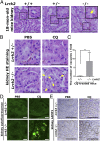
Leucine-rich repeat kinase 2 (LRRK2) has been associated with a variety of human diseases, including Parkinson's disease and Crohn's disease, whereas LRRK2 deficiency leads to accumulation of abnormal lysosomes in aged animals. However, the cellular roles and mechanisms of LRRK2-mediated lysosomal regulation have remained elusive. Here, we reveal a mechanism of stress-induced lysosomal response by LRRK2 and its target Rab GTPases. Lysosomal overload stress induced the recruitment of endogenous LRRK2 onto lysosomal membranes and activated LRRK2. An upstream adaptor Rab7L1 (Rab29) promoted the lysosomal recruitment of LRRK2. Subsequent family-wide screening of Rab GTPases that may act downstream of LRRK2 translocation revealed that Rab8a and Rab10 were specifically accumulated on overloaded lysosomes dependent on their phosphorylation by LRRK2. Rab7L1-mediated lysosomal targeting of LRRK2 attenuated the stress-induced lysosomal enlargement and promoted lysosomal secretion, whereas Rab8 stabilized by LRRK2 on stressed lysosomes suppressed lysosomal enlargement and Rab10 promoted lysosomal secretion, respectively. These effects were mediated by the recruitment of Rab8/10 effectors EHBP1 and EHBP1L1. LRRK2 deficiency augmented the chloroquine-induced lysosomal vacuolation of renal tubules in vivo. These results implicate the stress-responsive machinery composed of Rab7L1, LRRK2, phosphorylated Rab8/10, and their downstream effectors in the maintenance of lysosomal homeostasis.
Keywords: LRRK2; Rab GTPase; lysosome; phosphorylation.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Paisán-Ruíz C, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. - PubMed
-
- Zimprich A, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. - PubMed
-
- Lill CM, et al. 23andMe Genetic Epidemiology of Parkinson’s Disease Consortium; International Parkinson’s Disease Genomics Consortium; Parkinson’s Disease GWAS Consortium; Wellcome Trust Case Control Consortium 2) Comprehensive research synopsis and systematic meta-analyses in Parkinson’s disease genetics: The PDGene database. PLoS Genet. 2012;8:e1002548. - PMC - PubMed
-
- Zhang FR, et al. Genomewide association study of leprosy. N Engl J Med. 2009;361:2609–2618. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

