Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion
- PMID: 30209264
- PMCID: PMC6135805
- DOI: 10.1038/s41467-018-05853-7
Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion
Erratum in
-
Author Correction: Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion.Nat Commun. 2019 Jan 17;10(1):372. doi: 10.1038/s41467-019-08400-0. Nat Commun. 2019. PMID: 30655540 Free PMC article.
Abstract
Human antibody-secreting cells (ASC) in peripheral blood are found after vaccination or infection but rapidly apoptose unless they migrate to the bone marrow (BM). Yet, elements of the BM microenvironment required to sustain long-lived plasma cells (LLPC) remain elusive. Here, we identify BM factors that maintain human ASC > 50 days in vitro. The critical components of the cell-free in vitro BM mimic consist of products from primary BM mesenchymal stromal cells (MSC), a proliferation-inducing ligand (APRIL), and hypoxic conditions. Comparative analysis of protein-protein interactions between BM-MSC proteomics with differential RNA transcriptomics of blood ASC and BM LLPC identify two major survival factors, fibronectin and YWHAZ. The MSC secretome proteins and hypoxic conditions play a role in LLPC survival utilizing mechanisms that downregulate mTORC1 signaling and upregulate hypoxia signatures. In summary, we identify elements of the BM survival niche critical for maturation of blood ASC to BM LLPC.
Conflict of interest statement
F.E.-H.L. is the founder of MicroBplex, Inc., J.R. received grants from Stryker. A patent has been applied for by Emory University with F.E.L, I.S. and D.C. N. as named inventors. The patent application number is PCT/US2016/036650. The remaining authors declare no competing interests.
Figures
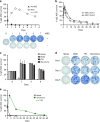
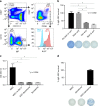



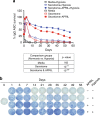
References
-
- Roldan E, Rodriguez C, Navas G, Parra C, Brieva JA. Cytokine network regulating terminal maturation of human bone marrow B cells capable of spontaneous and high rate Ig secretion in vitro. J. Immunol. 1992;149:2367–2371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI049660/AI/NIAID NIH HHS/United States
- R21 AI109601/AI/NIAID NIH HHS/United States
- U19 AI109962/AI/NIAID NIH HHS/United States
- R01 AI121252/AI/NIAID NIH HHS/United States
- P30 CA013148/CA/NCI NIH HHS/United States
- P01 AI125180/AI/NIAID NIH HHS/United States
- R01 DK109508/DK/NIDDK NIH HHS/United States
- N01 AI050029/AI/NIAID NIH HHS/United States
- P01 AI078907/AI/NIAID NIH HHS/United States
- HHSN266200500030C/AI/NIAID NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- U01 AI045969/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

