IL-27 amplifies cytokine responses to Gram-negative bacterial products and Salmonella typhimurium infection
- PMID: 30209294
- PMCID: PMC6135775
- DOI: 10.1038/s41598-018-32007-y
IL-27 amplifies cytokine responses to Gram-negative bacterial products and Salmonella typhimurium infection
Abstract
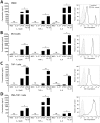
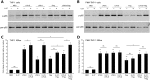
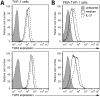
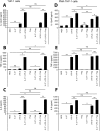
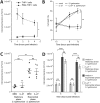
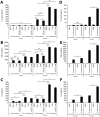
Cytokine responses from monocytes and macrophages exposed to bacteria are of particular importance in innate immunity. Focusing on the impact of the immunoregulatory cytokine interleukin (IL)-27 on control of innate immune system responses, we examined human immune responses to bacterial products and bacterial infection by E. coli and S. typhimurium. Since the effect of IL-27 treatment in human myeloid cells infected with bacteria is understudied, we treated human monocytes and macrophages with IL-27 and either LPS, flagellin, or bacteria, to investigate the effect on inflammatory signaling and cytokine responses. We determined that simultaneous stimulation with IL-27 and LPS derived from E. coli or S. typhimurium resulted in enhanced IL-12p40, TNF-α, and IL-6 expression compared to that by LPS alone. To elucidate if IL-27 manipulated the cellular response to infection with bacteria, we infected IL-27 treated human macrophages with S. typhimurium. While IL-27 did not affect susceptibility to S. typhimurium infection or S. typhimurium-induced cell death, IL-27 significantly enhanced proinflammatory cytokine production in infected cells. Taken together, we highlight a role for IL-27 in modulating innate immune responses to bacterial infection.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

