Long-Term Testosterone Therapy in Type 2 Diabetes Is Associated with Decreasing Waist Circumference and Improving Erectile Function
- PMID: 30209900
- PMCID: PMC6920065
- DOI: 10.5534/wjmh.180052M
Long-Term Testosterone Therapy in Type 2 Diabetes Is Associated with Decreasing Waist Circumference and Improving Erectile Function
Abstract
Purpose: To describe the 4-year metabolic follow-up results from the BLAST study.
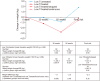
Materials and methods: Baseline hemoglobin A1c (HbA1c), weight, and waist circumference (WC) data were recorded in 185 men recruited for the BLAST randomised controlled trial (RCT) and erectile function (EF) scores were also available in an additional 48 men screened for the RCT. Intra/inter-group associations between these parameters and testosterone replacement therapy (TRT) were assessed at 1) end of the RCT (30 weeks), 2) open-label phase (82 weeks), and 3) final assessment via non-parametric statistics.
Results: Improvement in HbA1c and weight at the end of the RCT and open-label phase in men on TRT was not maintained long-term. The convergence in HbA1c could have been due to incentivised care with HbA1c targets. Interestingly those on TRT at final assessment required fewer anti-diabetic agents. The weight increase in routine care may have been due to changes in diabetes medication or an increase in lean muscle mass. WC continued to decrease in men on TRT indicating possible reduction in visceral fat. Improvement in EF scores continued with long-term TRT, this was abolished when TRT was discontinued.
Conclusions: This study hints at benefits in glycaemic control, weight and WC, and long-term RCTs studying mechanisms of benefit and clinical outcomes are necessary. Our results also show that EF scores continued to improve with long-term TRT, even beyond the 6 months that we previously reported in the BLAST RCT.
Keywords: Diabetes mellitus, type 2; Erectile dysfunction; Hypogonadism; Testosterone.
Copyright © 2020 Korean Society for Sexual Medicine and Andrology.
Conflict of interest statement
Professor Geoffrey Hackett has received honoraria for acting as a speaker for Bayer plc who provided the grant. Professor Sudarshan Ramachandran has received educational grants to attend meetings and honoraria for serving as a speaker for Besins Health Care Ltd. Professor Geoffrey Hackett has spoken at various national and international meetings on testosterone and PDE5I treatments in men. These companies and activities had no influence on this project. And other authors have no potential conflicts of interest to disclose.
Figures




References
-
- Hackett G, Cole N, Deshpande A, Popple M, Kennedy D, Wilkinson P. Biochemical hypodonadism and type 2 diabetes in primary care. Br J Diab Vasc Dis. 2009;9:226–231.
-
- Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring) 2013;21:1975–1981. - PubMed
-
- Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P, et al. The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study) Int J Clin Pract. 2014;68:203–215. - PubMed
-
- Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014;11:840–856. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

