TASK1 and TASK3 Are Coexpressed With ASIC1 in the Ventrolateral Medulla and Contribute to Central Chemoreception in Rats
- PMID: 30210304
- PMCID: PMC6123564
- DOI: 10.3389/fncel.2018.00285
TASK1 and TASK3 Are Coexpressed With ASIC1 in the Ventrolateral Medulla and Contribute to Central Chemoreception in Rats
Abstract
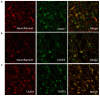
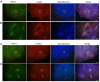
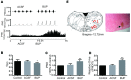
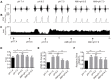
The ventrolateral medulla (VLM), including the lateral paragigantocellular nucleus (LPGi) and rostral VLM (RVLM), is commonly considered to be a chemosensitive region. However, the specific mechanism of chemoreception in the VLM remains elusive. Acid-sensing ion channels (ASICs), a family of voltage-independent proton-gated cation channels, can be activated by an external pH decrease to cause Na+ entry and induce neuronal excitability. TWIK-related acid-sensitive potassium channels (TASKs) are members of another group of pH-sensitive channels; in contrast to AISICs, they can be stimulated by pH increases and are inhibited by pH decreases in the physiological range. Our previous study demonstrated that ASICs take part in chemoreception. The aims of this study are to explore whether TASKs participate in the acid sensitivity of neurons in the VLM, thereby cooperating with ASICs. Our research demonstrated that TASKs, including TASK1 and TASK3, are colocalized with ASIC1 in VLM neurons. Blocking TASKs by microinjection of the non-selective TASK antagonist bupivacaine (BUP), specific TASK1 antagonist anandamide (AEA) or specific TASK3 antagonist ruthenium red (RR) into the VLM increased the integrated phrenic nerve discharge (iPND), shortened the inspiratory time (Ti) and enhanced the respiratory drive (iPND/Ti). In addition, microinjection of artificial cerebrospinal fluid (ACSF) at a pH of 7.0 or 6.5 prolonged Ti, increased iPND and enhanced respiratory drive, which were inhibited by the ASIC antagonist amiloride (AMI). By contrast, microinjection of alkaline ACSF decreased iPND and respiratory drive, which were inhibited by AEA. Taken together, our data suggest that TASK1 and TASK3 are coexpressed with ASIC1 in the VLM. Moreover, TASK1 and TASK3 contribute to the central regulation of breathing by coordinating with each other to perceive local pH changes; these results indicate a novel chemosensitive mechanism of the VLM.
Keywords: TASK1; TASK3; chemoreception; pH-sensitive; ventrolateral medulla.
Figures


Similar articles
-
Acid-sensing ion channels are expressed in the ventrolateral medulla and contribute to central chemoreception.Sci Rep. 2016 Dec 9;6:38777. doi: 10.1038/srep38777. Sci Rep. 2016. PMID: 27934921 Free PMC article.
-
TASK1 and TASK3 in orexin neuron of lateral hypothalamus contribute to respiratory chemoreflex by projecting to nucleus tractus solitarius.FASEB J. 2021 May;35(5):e21532. doi: 10.1096/fj.202002189R. FASEB J. 2021. PMID: 33817828
-
Acid sensing ion channel 1 in lateral hypothalamus contributes to breathing control.PLoS One. 2012;7(7):e39982. doi: 10.1371/journal.pone.0039982. Epub 2012 Jul 6. PLoS One. 2012. PMID: 22792205 Free PMC article.
-
TASK channels: channelopathies, trafficking, and receptor-mediated inhibition.Pflugers Arch. 2020 Jul;472(7):911-922. doi: 10.1007/s00424-020-02403-3. Epub 2020 May 29. Pflugers Arch. 2020. PMID: 32472332 Review.
-
Role of ventrolateral medulla in regulation of respiratory and cardiovascular systems.J Appl Physiol (1985). 1986 Oct;61(4):1249-63. doi: 10.1152/jappl.1986.61.4.1249. J Appl Physiol (1985). 1986. PMID: 3536832 Review.
Cited by
-
Advances in the Understanding of Two-Pore Domain TASK Potassium Channels and Their Potential as Therapeutic Targets.Molecules. 2022 Nov 28;27(23):8296. doi: 10.3390/molecules27238296. Molecules. 2022. PMID: 36500386 Free PMC article. Review.
-
The integrated brain network that controls respiration.Elife. 2023 Mar 8;12:e83654. doi: 10.7554/eLife.83654. Elife. 2023. PMID: 36884287 Free PMC article. Review.
-
Two central pattern generators from the crab, Cancer borealis, respond robustly and differentially to extreme extracellular pH.Elife. 2018 Dec 28;7:e41877. doi: 10.7554/eLife.41877. Elife. 2018. PMID: 30592258 Free PMC article.
-
Expression of Proton-Sensitive GPR31, GPR151, TASK1 and TASK3 in Common Skin Tumors.Cells. 2021 Dec 23;11(1):27. doi: 10.3390/cells11010027. Cells. 2021. PMID: 35011589 Free PMC article.
-
The "TASK" of Breathing: Anesthetic Relevance of Background Two-Pore Domain Potassium Channels as Therapeutic Targets for Respiratory Control.Anesth Analg. 2025 Feb 13;140(6):1414-25. doi: 10.1213/ANE.0000000000007365. Online ahead of print. Anesth Analg. 2025. PMID: 39946305 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

