Optogenetic Stimulation of GABAergic Neurons in the Globus Pallidus Produces Hyperkinesia
- PMID: 30210317
- PMCID: PMC6119815
- DOI: 10.3389/fnbeh.2018.00185
Optogenetic Stimulation of GABAergic Neurons in the Globus Pallidus Produces Hyperkinesia
Abstract
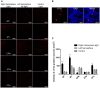
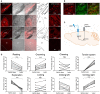
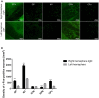
The globus pallidus (GP) is emerging as a critical locus of basal ganglia control of motor activity, but the exact role of GABAergic GP neurons remain to be defined. By targeted expression of channelrhodopsin 2 (ChR2) in GABAergic neurons using the VGAT-ChR2-EYFP transgenic mice, we showed that optogenetic stimulation of GABAergic neurons in the right GP produced hyperkinesia. Optogenetic stimulation of GABAergic GP neurons increased c-Fos-positive cells in GP, M1 cortex, and caudate-putamen (CPu), and decreased c-Fos-positive cells in entopeduncular nucleus (EPN), compared to the contralateral hemisphere. In agreement with the canonical basal ganglia model. Furthermore, we delivered AAV-CaMKIIα-ChR2-mCherry virus to the excitatory neurons of the subthalamic nucleus (STN) and selectively stimulated glutamatergic afferent fibers from the STN onto the GP. This optogenetic stimulation produced abnormal movements, similar to the behaviors that observed in the VGAT-ChR2-EYFP transgenic mice. Meanwhile, we found that the c-Fos expression pattern in the GP, M1, STN, EPN, and CPu produced by optogenetic activation of glutamatergic afferent fibers from the STN in GP was similar to the c-Fos expression pattern in the VGAT-ChR2-EYFP transgenic mice. Taken together, our results suggest that excess GP GABAergic neurons activity could be the neural substrate of abnormal involuntary movements in hyperkinetic movement disorders. The neural circuitry underlying the abnormal involuntary movements is associated with excessive GP, M1, CPu activity, and reduced EPN activity. Inhibition of GP GABAergic neurons represents new treatment targets for hyperkinetic movement disorder.
Keywords: GABAergic neurons; globus pallidus; hyperkinesia; movement disorders; optogenetic stimulation.
Figures

Similar articles
-
Optogenetic Activation of the Sensorimotor Cortex Reveals "Local Inhibitory and Global Excitatory" Inputs to the Basal Ganglia.Cereb Cortex. 2017 Dec 1;27(12):5716-5726. doi: 10.1093/cercor/bhx234. Cereb Cortex. 2017. PMID: 29028940
-
Subthalamic neurons coordinate basal ganglia function through differential neural pathways.J Neurosci. 2005 Aug 24;25(34):7743-53. doi: 10.1523/JNEUROSCI.1904-05.2005. J Neurosci. 2005. PMID: 16120775 Free PMC article.
-
High-frequency electrical stimulation of the subthalamic nucleus excites target structures in a model using c-fos immunohistochemistry.Neuroscience. 2014 Jun 13;270:212-25. doi: 10.1016/j.neuroscience.2014.04.016. Epub 2014 Apr 19. Neuroscience. 2014. PMID: 24755486
-
GABAergic control of the subthalamic nucleus.Prog Brain Res. 2007;160:173-88. doi: 10.1016/S0079-6123(06)60010-1. Prog Brain Res. 2007. PMID: 17499114 Review.
-
Globus pallidus internal segment.Prog Brain Res. 2007;160:135-50. doi: 10.1016/S0079-6123(06)60008-3. Prog Brain Res. 2007. PMID: 17499112 Review.
Cited by
-
Circuits for State-Dependent Modulation of Locomotion.Front Hum Neurosci. 2021 Nov 10;15:745689. doi: 10.3389/fnhum.2021.745689. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34858153 Free PMC article. Review.
-
Inhibitory basal ganglia nuclei differentially innervate pedunculopontine nucleus subpopulations and evoke differential motor and valence behaviors.bioRxiv [Preprint]. 2025 Apr 19:2024.08.05.606694. doi: 10.1101/2024.08.05.606694. bioRxiv. 2025. Update in: Elife. 2025 Aug 20;13:RP102308. doi: 10.7554/eLife.102308. PMID: 39149277 Free PMC article. Updated. Preprint.
-
AAVS1-targeted, stable expression of ChR2 in human brain organoids for consistent optogenetic control.Bioeng Transl Med. 2024 Jun 9;9(6):e10690. doi: 10.1002/btm2.10690. eCollection 2024 Nov. Bioeng Transl Med. 2024. PMID: 39545087 Free PMC article.
-
Optogenetic stimulation in the medial prefrontal cortex modulates stimulus valence from rewarding and aversive to neutral states.Front Psychiatry. 2023 Apr 11;14:1119803. doi: 10.3389/fpsyt.2023.1119803. eCollection 2023. Front Psychiatry. 2023. PMID: 37113545 Free PMC article.
-
Apelin-13 regulates electrical activity in the globus pallidus and induces postural changes in rats.Neural Regen Res. 2021 Nov;16(11):2264-2268. doi: 10.4103/1673-5374.310694. Neural Regen Res. 2021. PMID: 33818511 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

