Plasticity of Spine Structure: Local Signaling, Translation and Cytoskeletal Reorganization
- PMID: 30210329
- PMCID: PMC6123351
- DOI: 10.3389/fnsyn.2018.00029
Plasticity of Spine Structure: Local Signaling, Translation and Cytoskeletal Reorganization
Abstract
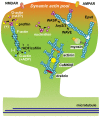
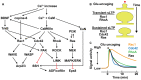
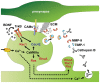
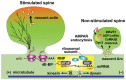
Dendritic spines are small protrusive structures on dendritic surfaces, and function as postsynaptic compartments for excitatory synapses. Plasticity of spine structure is associated with many forms of long-term neuronal plasticity, learning and memory. Inside these small dendritic compartments, biochemical states and protein-protein interactions are dynamically modulated by synaptic activity, leading to the regulation of protein synthesis and reorganization of cytoskeletal architecture. This in turn causes plasticity of structure and function of the spine. Technical advances in monitoring molecular behaviors in single dendritic spines have revealed that each signaling pathway is differently regulated across multiple spatiotemporal domains. The spatial pattern of signaling activity expands from a single spine to the nearby dendritic area, dendritic branch and the nucleus, regulating different cellular events at each spatial scale. Temporally, biochemical events are typically triggered by short Ca2+ pulses (~10-100 ms). However, these signals can then trigger activation of downstream protein cascades that can last from milliseconds to hours. Recent imaging studies provide many insights into the biochemical processes governing signaling events of molecular assemblies at different spatial localizations. Here, we highlight recent findings of signaling dynamics during synaptic plasticity and discuss their roles in long-term structural plasticity of dendritic spines.
Keywords: CaMKII; FRET FLIM; LTP; actin cytoskeleton; calcium signaling; dendritic spine; small GTPase; synaptic plasticity.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

