Pioglitazone, a PPAR-γ Activator, Stimulates BKCa but Suppresses IK M in Hippocampal Neurons
- PMID: 30210346
- PMCID: PMC6123368
- DOI: 10.3389/fphar.2018.00977
Pioglitazone, a PPAR-γ Activator, Stimulates BKCa but Suppresses IK M in Hippocampal Neurons
Abstract
Pioglitazone (PIO), a thiazolidinedone, was reported to stimulate peroxisome proliferator-activated receptor-γ (PPAR-γ) with anti-inflammatory, anti-proliferative, anti-diabetic, and antidepressive activities. However, whether this compound exerts any perturbations on Ca2+-activated K+ and M-type K+ currents in central neurons remains largely unresolved. In this study, we investigated the effects of PIO on these potassium currents in hippocampal neurons (mHippoE-14). In whole-cell current recordings, the presence of PIO (10 μM) increased the amplitude of Ca2+-activated K+ current [IK(Ca)] in mHippoE-14 cells. PIO-induced stimulation of IK(Ca) observed in these cells was reversed by subsequent addition of paxilline, yet not by TRAM-39 or apamin. In inside-out current recordings, PIO applied to the bath concentration-dependently increased the activity of large-conductance Ca2+-activated K+ (BKCa) channels with an EC50 value of 7.6 μM. Its activation of BKCa channels in mHippoE-14 cells was voltage-dependent and accompanied by both a lengthening in mean open time and a shortening in slow component of mean closed time. The activation curve of BKCa channels after addition of PIO was shifted to less depolarized potential without any change in the gating charge. PIO also suppressed the amplitude of M-type K+ currents inherently in mHippoE-14 neurons. Taken together, in addition to its agonistic action on PPAR-γ, PIO-induced perturbation of these potassium channels may be responsible for its widely pharmacological actions on hippocampal neurons.
Keywords: Ca2+-activated K+ current; M-type K+ current; hippocampal neuron; large-conductance Ca2+-activated K+ channel; pioglitazone.
Figures
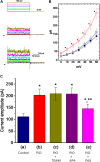
 ), during the exposure (
), during the exposure ( ) to 10 μM PIO and after washout (
) to 10 μM PIO and after washout ( ) of PIO (mean ± SEM; n = 11 for each point). ∗Significantly different from control groups taken at the same level of voltage pulse. (C) Bar graph showing summary of the effect of PIO, PIO plus TRAM-39, PIO plus apamin, and PIO plus paxilline, and PIO plus tolbutamide on IK(Ca) amplitude (mean ± SEM; n = 10–12 for each bar). Current amplitude was measured at +50 mV. (a) Control; (b) 10 μM PIO; (c) 10 μM PIO plus 3 μM TRAM-39; (d) 10 μM PIO plus 200 nM apamin; (e) 10 μM PIO plus 1 μM paxilline; (f) 10 μM PIO plus 30 μM tolbutamide. ∗Significantly different from control (P < 0.05) and ∗∗significantly different from PIO alone group (P < 0.05) (n = 9–10 for each bar).
) of PIO (mean ± SEM; n = 11 for each point). ∗Significantly different from control groups taken at the same level of voltage pulse. (C) Bar graph showing summary of the effect of PIO, PIO plus TRAM-39, PIO plus apamin, and PIO plus paxilline, and PIO plus tolbutamide on IK(Ca) amplitude (mean ± SEM; n = 10–12 for each bar). Current amplitude was measured at +50 mV. (a) Control; (b) 10 μM PIO; (c) 10 μM PIO plus 3 μM TRAM-39; (d) 10 μM PIO plus 200 nM apamin; (e) 10 μM PIO plus 1 μM paxilline; (f) 10 μM PIO plus 30 μM tolbutamide. ∗Significantly different from control (P < 0.05) and ∗∗significantly different from PIO alone group (P < 0.05) (n = 9–10 for each bar).
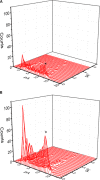
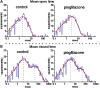
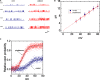
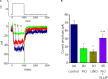
 ) and presence (
) and presence ( ) of 10 μM PIO is nearly identical. Each point represents mean ± SEM (n = 9–10). The dashed red lines obtained with or without addition of PIO are pointed toward the values of the reversal potential (i.e., 0.0 ± 0.1 mV, n = 8). (C) The relationship between relative open probability of BKCa channels and membrane potential obtained with or without addition of 10 μM PIO. The ramp pulses were applied from 0 to +80 mV with a duration of 1 s. Under inside-out current recordings, PIO (10 μM) was applied to the intracellular surface of the excised patch. The smooth lines represent the best fit to the Boltzmann equation as detailed in Section “Materials and Methods.”
) of 10 μM PIO is nearly identical. Each point represents mean ± SEM (n = 9–10). The dashed red lines obtained with or without addition of PIO are pointed toward the values of the reversal potential (i.e., 0.0 ± 0.1 mV, n = 8). (C) The relationship between relative open probability of BKCa channels and membrane potential obtained with or without addition of 10 μM PIO. The ramp pulses were applied from 0 to +80 mV with a duration of 1 s. Under inside-out current recordings, PIO (10 μM) was applied to the intracellular surface of the excised patch. The smooth lines represent the best fit to the Boltzmann equation as detailed in Section “Materials and Methods.”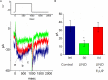
Similar articles
-
Characterization of Effectiveness in Concerted Ih Inhibition and IK(Ca) Stimulation by Pterostilbene (Trans-3,5-dimethoxy-4'-hydroxystilbene), a Stilbenoid.Int J Mol Sci. 2020 Jan 5;21(1):357. doi: 10.3390/ijms21010357. Int J Mol Sci. 2020. PMID: 31948124 Free PMC article.
-
High ability of zileuton ((±)-1-(1-benzo[b]thien-2-ylethyl)-1-hydroxyurea) to stimulate IK(Ca) but suppress IK(DR) and IK(M) independently of 5-lipoxygenase inhibition.Eur J Pharmacol. 2020 Nov 15;887:173482. doi: 10.1016/j.ejphar.2020.173482. Epub 2020 Aug 12. Eur J Pharmacol. 2020. PMID: 32795513
-
Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels.J Cell Physiol. 2017 Dec;232(12):3409-3421. doi: 10.1002/jcp.25788. Epub 2017 Apr 10. J Cell Physiol. 2017. PMID: 28075010
-
Inhibitory actions by ibandronate sodium, a nitrogen-containing bisphosphonate, on calcium-activated potassium channels in Madin-Darby canine kidney cells.Toxicol Rep. 2015 Aug 28;2:1182-1193. doi: 10.1016/j.toxrep.2015.08.010. eCollection 2015. Toxicol Rep. 2015. PMID: 28962460 Free PMC article.
-
High Efficacy by GAL-021: A Known Intravenous Peripheral Chemoreceptor Modulator that Suppresses BKCa-Channel Activity and Inhibits IK(M) or Ih.Biomolecules. 2020 Jan 25;10(2):188. doi: 10.3390/biom10020188. Biomolecules. 2020. PMID: 31991782 Free PMC article.
Cited by
-
Effective Activation by Kynurenic Acid and Its Aminoalkylated Derivatives on M-Type K+ Current.Int J Mol Sci. 2021 Jan 28;22(3):1300. doi: 10.3390/ijms22031300. Int J Mol Sci. 2021. PMID: 33525680 Free PMC article.
-
Channels that Cooperate with TRPV4 in the Brain.J Mol Neurosci. 2020 Nov;70(11):1812-1820. doi: 10.1007/s12031-020-01574-z. Epub 2020 Jun 10. J Mol Neurosci. 2020. PMID: 32524421 Review.
-
Peroxisome Proliferator-activated Receptor (PPAR)-γ Modifies Aβ Neurotoxin-induced Electrophysiological Alterations in Rat Primary Cultured Hippocampal Neurons.Iran J Pharm Res. 2019 Summer;18(3):1403-1418. doi: 10.22037/ijpr.2019.1100783. Iran J Pharm Res. 2019. PMID: 32641950 Free PMC article.
-
The Novel Direct Modulatory Effects of Perampanel, an Antagonist of AMPA Receptors, on Voltage-Gated Sodium and M-type Potassium Currents.Biomolecules. 2019 Oct 22;9(10):638. doi: 10.3390/biom9100638. Biomolecules. 2019. PMID: 31652643 Free PMC article.
-
Characterization of Effectiveness in Concerted Ih Inhibition and IK(Ca) Stimulation by Pterostilbene (Trans-3,5-dimethoxy-4'-hydroxystilbene), a Stilbenoid.Int J Mol Sci. 2020 Jan 5;21(1):357. doi: 10.3390/ijms21010357. Int J Mol Sci. 2020. PMID: 31948124 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

