Imatinib Inhibits GH Secretion From Somatotropinomas
- PMID: 30210447
- PMCID: PMC6120347
- DOI: 10.3389/fendo.2018.00453
Imatinib Inhibits GH Secretion From Somatotropinomas
Abstract
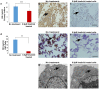
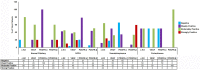
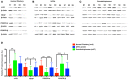
Background: Imatinib, a tyrosine kinase inhibitor, causes growth failure in children with chronic myeloid leukemia probably by targeting the growth hormone (GH)/insulin like growth factor-1 (IGF-1) axis. We aim to explore the imatinib targets expression in pituitary adenomas and study the effect of imatinib on GH secretion in somatotropinoma cells and GH3 cell line. Materials and Methods: The expression pattern of imatinib's targets (c-kit, VEGF, and PDGFR-α/β) was studied using immunohistochemistry and immunoblotting 157 giant (≥4 cm) pituitary adenomas (121 non-functioning pituitary adenomas, 32 somatotropinomas, and four prolactinomas) and compared to normal pituitary (n = 4) obtained at autopsy. The effect imatinib on GH secretion, cell viability, immunohistochemistry, electron microscopy, and apoptosis was studied in primary culture of human somatotropinomas (n = 20) and in rat somato-mammotroph GH3 cell-line. A receptor tyrosine kinase array was applied to human samples to identify altered pathways. Results: Somatotropinomas showed significantly higher immunopositivity for c-kit and platelet-derived growth factor receptor-β (PDGFR-β; P < 0.009 and P < 0.001, respectively), while staining for platelet-derived growth factor receptor-α (PDGFR-α) and vascular endothelial growth factor (VEGF) revealed a weaker expression (P < 0.001) compared to normal pituitary. Imatinib inhibited GH secretion from both primary culture (P < 0.01) and GH3 cells (P < 0.001), while it did not affect cell viability and apoptosis. The receptor tyrosine kinase array showed that imatinib inhibits GH signaling via PDGFR-β pathway. Conclusion: Imatinib inhibits GH secretion in somatotropinoma cells without affecting cell viability and may be used as an adjunct therapy for treating GH secreting pituitary adenomas.
Keywords: PDGFR-α/β; VEGF; c-kit; growth hormone; imatinib; somatotropinoma.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

