Phosphorus and Nitrogen Drive the Seasonal Dynamics of Bacterial Communities in Pinus Forest Rhizospheric Soil of the Qinling Mountains
- PMID: 30210463
- PMCID: PMC6119707
- DOI: 10.3389/fmicb.2018.01930
Phosphorus and Nitrogen Drive the Seasonal Dynamics of Bacterial Communities in Pinus Forest Rhizospheric Soil of the Qinling Mountains
Abstract
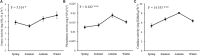
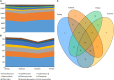
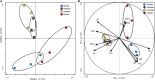
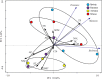
The temporal distribution patterns of bacterial communities, as an important group in mountain soil, are affected by various environmental factors. To improve knowledge regarding the successional seasonal dynamics of the mountain soil bacterial communities, the rhizospheric soil of a 30-year-old natural secondary Pinus tabulaeformis forest, located in the high-altitude (1900 m a.s.l.) of the temperate Qinling Mountains, was sampled and studied during four different seasons. The bacterial community composition and structure in the rhizospheric soil were studied using an Illumina MiSeq Sequencing platform. Furthermore, the edaphic properties and soil enzymatic activities (urease, phosphatase, and catalase) were measured in order to identify the main impact factors on the soil bacterial community. According to the results, all of the edaphic properties and soil enzymatic activities were significantly affected by the seasonal changes, except for the C/N ratio. Although the biomasses of soil bacterial communities increased during the summer and autumn (warm seasons), their Shannon diversity and Pielou's evenness were decreased. Proteobacteria, Acidobacteria, Actinobacteria, Planctomycetes, and Bacteroidetes were the predominant bacterial groups in all of the soil samples, and the genera of Ktedonobacter, Sphingobium as well as an unclassified member of the Ktedonobacteria were the keystone taxa. The composition and structure of soil bacterial communities were strongly impacted by the edaphic properties, especially the temperature, moisture, ammoniacal nitrogen, available phosphorus and total phosphorus which were the crucial factors to drive the temporal distribution of the soil bacterial community and diversity. In conclusion, the soil temperature, moisture and the nutrients N and P were the crucial edaphic factors for shaping the rhizospheric soil bacterial communities as season and climate change in a P. tabulaeformis forest of Qinling Mountains.
Keywords: Pinus; bacterial community; biodiversity; rhizosphere soil; season dynamics.
Figures


References
-
- Alef K., Nannipieri P. (1995). Methods in Applied Soil Microbiology and Biochemistry. London: Academic Press.
-
- Allison S. D., Treseder K. K. (2008). Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Change Biol. 142898–2909. 10.1111/j.1365-2486.2008.01716.x - DOI
-
- An S., Huang Y., Zheng F. (2009). Evaluation of soil microbial indices along a revegetation chronosequence in grassland soils on the Loess Plateau, Northwest China. Appl. Soil Ecol. 41 286–292. 10.1016/j.apsoil.2008.12.001 - DOI
-
- Baas-Becking L. G. M. (1934). Geobiologie; of Inleiding tot de Milieukunde. ıOakland, CA: WP Van Stockum & Zoon NV.
LinkOut - more resources
Full Text Sources
Other Literature Sources

