Effect of Pressure, Reconstituted RTE Meat Microbiota, and Antimicrobials on Survival and Post-pressure Growth of Listeria monocytogenes on Ham
- PMID: 30210467
- PMCID: PMC6119701
- DOI: 10.3389/fmicb.2018.01979
Effect of Pressure, Reconstituted RTE Meat Microbiota, and Antimicrobials on Survival and Post-pressure Growth of Listeria monocytogenes on Ham
Abstract
Pressure treatment of ready-to-eat (RTE) meats extends the shelf life and reduces risks associated with Listeria monocytogenes. However, pressure reduces numbers of Listeria on ham by less than 5 log (CFU/g) and pressure effects on other meat microbiota are poorly documented. This study investigated the impact of pressure and RTE meat microbiota, with or without nisin and rosemary oil, on survival of Listeria after refrigerated storage. Ham was inoculated with a 5-strain cocktail of L. monocytogenes alone or with a cocktail of RTE meat microbiota consisting of Brochothrix thermosphacta, Carnobacterium maltaromaticum, Leuconostoc gelidum, and Lactobacillussakei. Products were treated at 500 MPa at 5°C for 1 or 3 min, with or without rosemary extract or nisin. Surviving cells were differentially enumerated after pressure treatment and after 4 weeks of refrigerated storage. After 4 weeks of storage, products were also analyzed by high throughput sequencing of 16S rRNA amplicons. Pressure treatment reduced counts of Listeria by 1 to 2 log (CFU/g); inactivation of RTE meat microbiota was comparable. Counts of Listeria increased by 1-3 log (CFU/g) during refrigerated storage. RTE meat microbiota did not influence pressure inactivation of Listeria but prevented growth of Listeria during refrigerated storage. Rosemary extract did not influence bacterial inactivation or growth. The combination of nisin with pressure treatment for 3 min reduced counts of Listeria and meat microbiota by >5 log (CFU/g); after 4 weeks of storage, counts were below the detection limit. In conclusion, pressure alone does not eliminate Listeria or other microbiota on RTE ham; however, the presence of non-pathogenic microbiota prevents growth of Listeria on pressure treated ham and has a decisive influence on post-pressure survival and growth.
Keywords: Lactobacillus sakei; Leuconostoc gelidum; Listeria monocytogenes; antimicrobials; high pressure processing; meat microbiota; nisin; ready-to-eat meat.
Figures
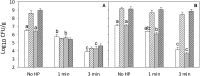
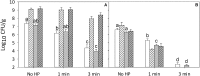
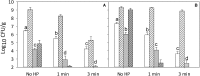
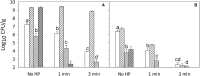
 ), or controls with 0.5% ethanol (•) or 0.02N HCl (
), or controls with 0.5% ethanol (•) or 0.02N HCl ( ), rosemary extract (
), rosemary extract ( ), nisin (
), nisin ( ); no pressure treatment (blue), 1 min at 500 MPa (red), 3 min at 500 MPa (black).
); no pressure treatment (blue), 1 min at 500 MPa (red), 3 min at 500 MPa (black).Similar articles
-
Antimicrobial activity of bioactive starch packaging films against Listeria monocytogenes and reconstituted meat microbiota on ham.Int J Food Microbiol. 2019 Sep 16;305:108253. doi: 10.1016/j.ijfoodmicro.2019.108253. Epub 2019 Jun 19. Int J Food Microbiol. 2019. PMID: 31233962
-
Effect of in-package atmospheric cold plasma discharge on microbial safety and quality of ready-to-eat ham in modified atmospheric packaging during storage.J Food Sci. 2020 Apr;85(4):1203-1212. doi: 10.1111/1750-3841.15072. Epub 2020 Mar 2. J Food Sci. 2020. PMID: 32118300
-
Non-Destructive Luminescence-Based Screening Tool for Listeria monocytogenes Growth on Ham.Foods. 2020 Nov 20;9(11):1700. doi: 10.3390/foods9111700. Foods. 2020. PMID: 33233500 Free PMC article.
-
Prevalence and challenge tests of Listeria monocytogenes in Belgian produced and retailed mayonnaise-based deli-salads, cooked meat products and smoked fish between 2005 and 2007.Int J Food Microbiol. 2009 Jul 31;133(1-2):94-104. doi: 10.1016/j.ijfoodmicro.2009.05.002. Epub 2009 May 9. Int J Food Microbiol. 2009. PMID: 19515447 Review.
-
Natural Antimicrobials for Listeria monocytogenes in Ready-to-Eat Meats: Current Challenges and Future Prospects.Microorganisms. 2023 May 16;11(5):1301. doi: 10.3390/microorganisms11051301. Microorganisms. 2023. PMID: 37317275 Free PMC article. Review.
Cited by
-
Multitarget preservation technologies for chemical-free sustainable meat processing.J Food Sci. 2022 Oct;87(10):4312-4328. doi: 10.1111/1750-3841.16329. Epub 2022 Sep 19. J Food Sci. 2022. PMID: 36120824 Free PMC article. Review.
-
Combination of High-Pressure Treatment at 500 MPa and Biopreservation with a Lactococcus lactis Strain for Lowering the Bacterial Growth during Storage of Diced Cooked Ham with Reduced Nitrite Salt.Microorganisms. 2022 Feb 16;10(2):456. doi: 10.3390/microorganisms10020456. Microorganisms. 2022. PMID: 35208910 Free PMC article.
-
Comprehensive Review on the Biocontrol of Listeria monocytogenes in Food Products.Foods. 2024 Feb 28;13(5):734. doi: 10.3390/foods13050734. Foods. 2024. PMID: 38472848 Free PMC article. Review.
-
Antimicrobial Activity of Oregano Essential Oil Incorporated in Sodium Alginate Edible Films: Control of Listeria monocytogenes and Spoilage in Ham Slices Treated with High Pressure Processing.Materials (Basel). 2019 Nov 12;12(22):3726. doi: 10.3390/ma12223726. Materials (Basel). 2019. PMID: 31718078 Free PMC article.
-
Listeria monocytogenes growth kinetics in refrigerated ready-to-eat dips and dip components.PLoS One. 2020 Jun 30;15(6):e0235472. doi: 10.1371/journal.pone.0235472. eCollection 2020. PLoS One. 2020. PMID: 32603372 Free PMC article.
References
-
- Abdollahzadeh E., Rezaei M., Hosseini H. (2014). Antibacterial activity of plant essential oils and extracts: the role of thyme essential oil, nisin, and their combination to control Listeria monocytogenes inoculated in minced fish meat. Food Control 35 177–183. 10.1016/j.foodcont.2013.07.004 - DOI
-
- Arqués J. L., Rodríguez E., Gaya P., Medina M., Nunez M. (2005). Effect of combinations of high-pressure treatment and bacteriocin-producing lactic acid bacteria on the survival of Listeria monocytogenes in raw milk cheese. Int. Dairy J. 15 893–900. 10.1016/j.idairyj.2004.07.020 - DOI
-
- Bull M. K., Hayman M. M., Stewart C. M., Szabo E. A., Knabel S. J. (2005). Effect of prior growth temperature, type of enrichment medium, and temperature and time of storage on recovery of Listeria monocytogenes following high pressure processing of milk. Int. J. Food Microbiol. 101 53–61. 10.1016/j.ijfoodmicro.2004.10.045 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

