Host Restriction Factors APOBEC3G/3F and Other Interferon-Related Gene Expressions Affect Early HIV-1 Infection in Northern Pig-Tailed Macaque (Macaca leonina)
- PMID: 30210504
- PMCID: PMC6120991
- DOI: 10.3389/fimmu.2018.01965
Host Restriction Factors APOBEC3G/3F and Other Interferon-Related Gene Expressions Affect Early HIV-1 Infection in Northern Pig-Tailed Macaque (Macaca leonina)
Abstract
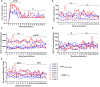
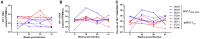
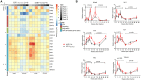
The northern pig-tailed macaques (NPMs) lack TRIM5α, an antiviral restriction factor, and instead have TRIM5-CypA. In our previous study, we demonstrated that HIV-1NL4-3 successfully infected NPMs and formed a long-term viral reservoir in vivo. However, the HIV-1-infected NPMs showed relatively high viremia in the first 6 weeks of infection, which declined thereafter suggesting that HIV-1 NL4-3 infection in these animals was only partly permissive. To optimize HIV-1 infection in NPMs therefore, we generated HIV-1NL4-R3A and stHIV-1sv, and infected NPMs with these viruses. HIV-1NL4-R3A and stHIV-1sv can replicate persistently in NPMs during 41 weeks of acute infection stage. Compared to the HIV-1NL4-R3A, stHIV-1sv showed a notably higher level of replication, and the NPMs infected with the latter induced a more robust neutralizing antibody but a weaker cellular immune response. In addition, IFN-I signaling was significantly up-regulated with the viral replication, and was higher in the stHIV-1sv infected macaques. Consequently, the sequences of pro-viral env showed fewer G-A hyper-mutations in stHIV-1sv, suggesting that vif gene of SIV could antagonize the antiviral effects of APOBEC3 proteins in NPMs. Taken together, NPMs infected with HIV-1NL4-R3A and stHIV-1sv show distinct virological and immunological features. Furthermore, interferon-related gene expression might play a role in controlling primary HIV-1NL4-R3A and stHIV-1sv replication in NPMs. This result suggests NPM is a potential HIV/AIDS animal model.
Keywords: APOBEC3; HIV-1NL4-R3A; IFN-I signaling; neutralizing antibodies; northern pig-tailed macaques; stHIV-1sv.
Figures



Similar articles
-
A Novel Vpu Adaptive Mutation of HIV-1 Degrades Tetherin in Northern Pig-Tailed Macaques (Macaca leonina) Mainly via the Ubiquitin-Proteasome Pathway and Increases Viral Release.J Virol. 2023 Apr 27;97(4):e0020023. doi: 10.1128/jvi.00200-23. Epub 2023 Mar 27. J Virol. 2023. PMID: 36971578 Free PMC article.
-
HIV-1 but not SIVmac239 induces higher interferon-α antiviral state in chronic infected northern pig-tailed macaques (Macaca leonina).Microbes Infect. 2022 Jul-Aug;24(5):104970. doi: 10.1016/j.micinf.2022.104970. Epub 2022 Mar 21. Microbes Infect. 2022. PMID: 35331910
-
Replication potentials of HIV-1/HSIV in PBMCs from northern pig-tailed macaque (Macaca leonina).Dongwuxue Yanjiu. 2014 May;35(3):186-95. doi: 10.11813/j.issn.0254-5853.2014.3.186. Dongwuxue Yanjiu. 2014. PMID: 24866489 Free PMC article.
-
Minimally Modified HIV-1 Infection of Macaques: Development, Utility, and Limitations of Current Models.Viruses. 2024 Oct 16;16(10):1618. doi: 10.3390/v16101618. Viruses. 2024. PMID: 39459950 Free PMC article. Review.
-
HIV and SIV infection: the role of cellular restriction and immune responses in viral replication and pathogenesis.APMIS. 2009 May;117(5-6):400-12. doi: 10.1111/j.1600-0463.2009.02450.x. APMIS. 2009. PMID: 19400864 Free PMC article. Review.
Cited by
-
An Alu Element Insertion in Intron 1 Results in Aberrant Alternative Splicing of APOBEC3G Pre-mRNA in Northern Pig-Tailed Macaques (Macaca leonina) That May Reduce APOBEC3G-Mediated Hypermutation Pressure on HIV-1.J Virol. 2020 Jan 31;94(4):e01722-19. doi: 10.1128/JVI.01722-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31776266 Free PMC article.
-
Transcription Factor ZNF683 Inhibits SIV/HIV Replication through Regulating IFNγ Secretion of CD8+ T Cells.Viruses. 2022 Mar 30;14(4):719. doi: 10.3390/v14040719. Viruses. 2022. PMID: 35458449 Free PMC article.
-
How Retroviruses and Retrotransposons in Our Genome May Contribute to Autoimmunity in Rheumatological Conditions.Front Immunol. 2020 Nov 13;11:593891. doi: 10.3389/fimmu.2020.593891. eCollection 2020. Front Immunol. 2020. PMID: 33281822 Free PMC article. Review.
-
CXCR4- and CCR5-Tropic HIV-1 Clones Are Both Tractable to Grow in Rhesus Macaques.Front Microbiol. 2018 Oct 18;9:2510. doi: 10.3389/fmicb.2018.02510. eCollection 2018. Front Microbiol. 2018. PMID: 30405570 Free PMC article.
-
Superior intestinal integrity and limited microbial translocation are associated with lower immune activation in SIVmac239-infected northern pig-tailed macaques (Macaca leonina).Zool Res. 2019 Nov 18;40(6):522-531. doi: 10.24272/j.issn.2095-8137.2019.047. Zool Res. 2019. PMID: 31033262 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

