Extracellular galectin-3 facilitates colon cancer cell migration and is related to the epidermal growth factor receptor
- PMID: 30210679
- PMCID: PMC6129507
Extracellular galectin-3 facilitates colon cancer cell migration and is related to the epidermal growth factor receptor
Abstract
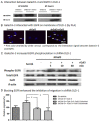
We previously found that galectin-3 enhanced DLD-1 cell migration through the K-Ras-Raf-Erk1/2 pathway, but the effect of extracellular galectin-3 on cancer cell migration and its interaction with the epidermal growth factor receptor (EGFR) remained unknown. We aimed to determine the effect of extracellular galectin-3 on colon cancer cell migration and its correlation with the EGFR expression. Western blotting was performed to analyze galectin-3 secretion, shRNA was used to stably knock down galectin-3 expression and a migration assay was performed to evaluate colon cancer cell migration. Tissues from eighty patients with four different stages of colon cancer were obtained and compared to normal colon tissue. The galectin-3 knockdown colon cancer cells exhibited decreased migration, which was restored by recombinant galectin-3. An EGFR blocking antibody decreased colon cancer cell migration. The addition of recombinant galectin-3 increased phosphorylated EGFR expression within minutes and enhanced the internalization of the EGFR from the cell membrane to the cytoplasm, particularly upon EGF stimulation. Extracellular galectin-3 increased colon cancer cell migration, which correlated with the EGFR. Targeting galectin-3 may have a synergistic effect on EGFR-targeted therapy.
Keywords: Colorectal carcinoma; EGFR; cell migration; galectin-3.
Figures






Similar articles
-
Overexpression of galectin-3 enhances migration of colon cancer cells related to activation of the K-Ras-Raf-Erk1/2 pathway.J Gastroenterol. 2013 Mar;48(3):350-9. doi: 10.1007/s00535-012-0663-3. Epub 2012 Sep 27. J Gastroenterol. 2013. PMID: 23015305
-
Galectin-8 expression decreases in cancer compared with normal and dysplastic human colon tissue and acts significantly on human colon cancer cell migration as a suppressor.Gut. 2002 Mar;50(3):392-401. doi: 10.1136/gut.50.3.392. Gut. 2002. PMID: 11839721 Free PMC article.
-
EGFR and HER2 exert distinct roles on colon cancer cell functional properties and expression of matrix macromolecules.Biochim Biophys Acta. 2014 Aug;1840(8):2651-61. doi: 10.1016/j.bbagen.2014.04.019. Epub 2014 May 2. Biochim Biophys Acta. 2014. PMID: 24792576
-
The ErbB/HER family of protein-tyrosine kinases and cancer.Pharmacol Res. 2014 Jan;79:34-74. doi: 10.1016/j.phrs.2013.11.002. Epub 2013 Nov 20. Pharmacol Res. 2014. PMID: 24269963 Review.
-
Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody.Drugs Today (Barc). 2005 Feb;41(2):107-27. doi: 10.1358/dot.2005.41.2.882662. Drugs Today (Barc). 2005. PMID: 15821783 Review.
Cited by
-
Pleiotropic Effects of Modified Citrus Pectin.Nutrients. 2019 Nov 1;11(11):2619. doi: 10.3390/nu11112619. Nutrients. 2019. PMID: 31683865 Free PMC article. Review.
-
Galectin‑3 facilitates the proliferation and migration of nasopharyngeal carcinoma cells via activation of the ERK1/2 and Akt signaling pathways, and is positively correlated with the inflammatory state of nasopharyngeal carcinoma.Mol Med Rep. 2021 May;23(5):370. doi: 10.3892/mmr.2021.12009. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760180 Free PMC article.
-
Galectins in epithelial-mesenchymal transition: roles and mechanisms contributing to tissue repair, fibrosis and cancer metastasis.Biol Res. 2024 Apr 4;57(1):14. doi: 10.1186/s40659-024-00490-5. Biol Res. 2024. PMID: 38570874 Free PMC article. Review.
-
The pleiotropic role of galectin-3 in melanoma progression: Unraveling the enigma.Adv Cancer Res. 2023;157:157-193. doi: 10.1016/bs.acr.2022.06.001. Epub 2022 Aug 8. Adv Cancer Res. 2023. PMID: 36725108 Free PMC article. Review.
-
Automatic target-seeking nanoparticle inhibiting orthotopic drug-resistant colon cancer and liver metastases via regulating cancer cell adhesion and proliferation.J Nanobiotechnology. 2025 Jun 6;23(1):423. doi: 10.1186/s12951-025-03422-x. J Nanobiotechnology. 2025. PMID: 40481460 Free PMC article.
References
-
- Mayoral MA, Mayoral C, Meneses A, Villalvazo L, Guzman A, Espinosa B, Ochoa JL, Zenteno E, Guevara J. Identification of galectin-3 and mucin-type O-glycans in breast cancer and its metastasis to brain. Cancer Invest. 2008;26:615–623. - PubMed
-
- Savin S, Cvejic D, Isic T, Paunovic I, Tatic S, Havelka M. Thyroid peroxidase and galectin-3 immunostaining in differentiated thyroid carcinoma with clinicopathologic correlation. Hum Pathol. 2008;39:1656–1663. - PubMed
-
- Schoeppner HL, Raz A, Ho SB, Bresalier RS. Expression of anendogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer. 1995;75:2818–2826. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
