Transcription factor Tbx18 induces the differentiation of c-kit+ canine mesenchymal stem cells (cMSCs) into SAN-like pacemaker cells in a co-culture model in vitro
- PMID: 30210689
- PMCID: PMC6129520
Transcription factor Tbx18 induces the differentiation of c-kit+ canine mesenchymal stem cells (cMSCs) into SAN-like pacemaker cells in a co-culture model in vitro
Abstract
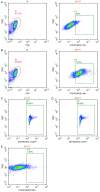
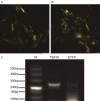
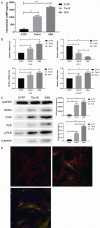
Bone mesenchymal stem cells (MSCs), as well as cardiomyocytes, are derived from early mesoderm, becoming committed to their fate under the influence of different differentiation factors. We examined whether the overexpression of Tbx18 can induce the differentiation of c-kit+ cMSCs into a phenotype similar to that of native pacemaker cells and whether these transfected cells can couple to adjacent atrial cells with functional consequences. The c-kit+ cMSCs were first sorted, then transfected with different lentiviral vectors. Tbx18-c-kit+ cMSCs represented the experimental group, while EYFP-c-kit+ cMSCs and canine sinoatrial node (SAN) cells were used as controls. Within days of transfection, the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel HCN4 protein and gap junction protein Connexin 45 (Cx45) expression in Tbx18-c-kit+ cMSCs were 12-fold and 5.6-fold higher, respectively, than that in EYFP-c-kit+ cMSCs. After co-culture with canine atrial cells in vitro for three days, the funny currents (If) were recorded in the Tbx18-c-kit+ cMSCs, but not in EYFP-c-kit+ cMSCs. The trend of these If currents was highly similar to that of SAN cells, although the current density was smaller. The Tbx18-EYFP-c-kit+ cMSCs showed responsiveness to β-adrenergic stimulation, and the intracellular cyclic adenosine monophosphate (cAMP) level was higher than that in EYFP-c-kit+ cMSCs. The Tbx18-EYFP-c-kit+ cMSCs delivered fluorescent dye to neighboring atrial cells via gap junctions, thus these cell pairs could communicate as a pacemaker unit. We propose that the overexpression of Tbx18 in c-kit+ cMSCs induces their differentiation to SAN-like pacemaker cells.
Keywords: HCN4; Tbx18; c-kit+ mesenchymal stem cells; co-culture; gap junction.
Conflict of interest statement
None.
Figures


Similar articles
-
Electric pulse current stimulation increases electrophysiological properties of If current reconstructed in mHCN4-transfected canine mesenchymal stem cells.Exp Ther Med. 2016 Apr;11(4):1323-1329. doi: 10.3892/etm.2016.3072. Epub 2016 Feb 11. Exp Ther Med. 2016. PMID: 27073443 Free PMC article.
-
Shox2 influences mesenchymal stem cell fate in a co-culture model in vitro.Mol Med Rep. 2016 Jul;14(1):637-42. doi: 10.3892/mmr.2016.5306. Epub 2016 May 18. Mol Med Rep. 2016. PMID: 27222368 Free PMC article.
-
Tbx18 promoted the conversion of human-induced pluripotent stem cell-derived cardiomyocytes into sinoatrial node-like pacemaker cells.Cell Biol Int. 2022 Mar;46(3):403-414. doi: 10.1002/cbin.11738. Epub 2021 Dec 30. Cell Biol Int. 2022. PMID: 34882885
-
Pacemaker activity of the human sinoatrial node: effects of HCN4 mutations on the hyperpolarization-activated current.Europace. 2014 Mar;16(3):384-95. doi: 10.1093/europace/eut348. Europace. 2014. PMID: 24569893 Review.
-
cyclic AMP Regulation and Its Command in the Pacemaker Channel HCN4.Front Physiol. 2020 Jul 7;11:771. doi: 10.3389/fphys.2020.00771. eCollection 2020. Front Physiol. 2020. PMID: 32733276 Free PMC article. Review.
Cited by
-
Conversion of Unmodified Stem Cells to Pacemaker Cells by Overexpression of Key Developmental Genes.Cells. 2023 May 13;12(10):1381. doi: 10.3390/cells12101381. Cells. 2023. PMID: 37408215 Free PMC article.
-
Shenfu Injection: A Famous Chinese Prescription That Promotes HCN4 Activity in Bone Marrow Mesenchymal Stem Cells.Evid Based Complement Alternat Med. 2021 Aug 17;2021:9912844. doi: 10.1155/2021/9912844. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34457032 Free PMC article.
-
G9a inhibition promotes the formation of pacemaker-like cells by reducing the enrichment of H3K9me2 in the HCN4 promoter region.Mol Med Rep. 2023 Feb;27(2):21. doi: 10.3892/mmr.2022.12908. Epub 2022 Dec 9. Mol Med Rep. 2023. PMID: 36484369 Free PMC article.
-
Congenital Disorders of the Human Urinary Tract: Recent Insights From Genetic and Molecular Studies.Front Pediatr. 2019 Apr 11;7:136. doi: 10.3389/fped.2019.00136. eCollection 2019. Front Pediatr. 2019. PMID: 31032239 Free PMC article. Review.
-
Genetically Modified Porcine Mesenchymal Stem Cells by Lentiviral Tbx18 Create a Biological Pacemaker.Stem Cells Int. 2019 Nov 7;2019:3621314. doi: 10.1155/2019/3621314. eCollection 2019. Stem Cells Int. 2019. PMID: 31814832 Free PMC article.
References
-
- Xu M, Wani M, Dai YS, Wang J, Yan M, Ayub A, Ashraf M. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658–2665. - PubMed
-
- Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
