A mononuclear nonheme {FeNO}6 complex: synthesis and structural and spectroscopic characterization
- PMID: 30210769
- PMCID: PMC6124912
- DOI: 10.1039/c8sc01962b
A mononuclear nonheme {FeNO}6 complex: synthesis and structural and spectroscopic characterization
Abstract
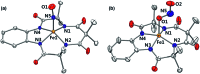
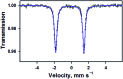
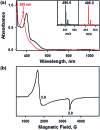
While the synthesis and characterization of {FeNO}7,8,9 complexes have been well documented in heme and nonheme iron models, {FeNO}6 complexes have been less clearly understood. Herein, we report the synthesis and structural and spectroscopic characterization of mononuclear nonheme {FeNO}6 and iron(iii)-nitrito complexes bearing a tetraamido macrocyclic ligand (TAML), such as [(TAML)FeIII(NO)]- and [(TAML)FeIII(NO2)]2-, respectively. First, direct addition of NO(g) to [FeIII(TAML)]- results in the formation of [(TAML)FeIII(NO)]-, which is sensitive to moisture and air. The spectroscopic data of [(TAML)FeIII(NO)]-, such as 1H nuclear magnetic resonance and X-ray absorption spectroscopies, combined with computational study suggest the neutral nature of nitric oxide with a diamagnetic Fe center (S = 0). We also provide alternative pathways for the generation of [(TAML)FeIII(NO)]-, such as the iron-nitrite reduction triggered by protonation in the presence of ferrocene, which acts as an electron donor, and the photochemical iron-nitrite reduction. To the best of our knowledge, the present study reports the first photochemical nitrite reduction in nonheme iron models.
Figures




Similar articles
-
Synthetic mononuclear nonheme iron-oxygen intermediates.Acc Chem Res. 2015 Aug 18;48(8):2415-23. doi: 10.1021/acs.accounts.5b00218. Epub 2015 Jul 23. Acc Chem Res. 2015. PMID: 26203519
-
Mechanistic Insight into the Nitric Oxide Dioxygenation Reaction of Nonheme Iron(III)-Superoxo and Manganese(IV)-Peroxo Complexes.Angew Chem Int Ed Engl. 2016 Sep 26;55(40):12403-7. doi: 10.1002/anie.201605705. Epub 2016 Sep 4. Angew Chem Int Ed Engl. 2016. PMID: 27593390 Free PMC article.
-
Catalytic Four-Electron Reduction of Dioxygen by Ferrocene Derivatives with a Nonheme Iron(III) TAML Complex.Inorg Chem. 2020 Dec 21;59(24):18010-18017. doi: 10.1021/acs.inorgchem.0c02400. Epub 2020 Dec 10. Inorg Chem. 2020. PMID: 33300784
-
High-valent iron complexes with tetraamido macrocyclic ligands: structures, Mössbauer spectroscopy, and DFT calculations.J Inorg Biochem. 2006 Apr;100(4):606-19. doi: 10.1016/j.jinorgbio.2005.12.016. Epub 2006 Feb 7. J Inorg Biochem. 2006. PMID: 16464502 Review.
-
Nitric oxide interaction with insect nitrophorins and thoughts on the electron configuration of the {FeNO}6 complex.J Inorg Biochem. 2005 Jan;99(1):216-36. doi: 10.1016/j.jinorgbio.2004.10.009. J Inorg Biochem. 2005. PMID: 15598503 Review.
Cited by
-
Acid-induced nitrite reduction of nonheme iron(ii)-nitrite: mimicking biological Fe-NiR reactions.Chem Sci. 2023 Feb 23;14(11):2935-2942. doi: 10.1039/d2sc06704h. eCollection 2023 Mar 15. Chem Sci. 2023. PMID: 36937601 Free PMC article.
-
Hemilabile Proton Relays and Redox Activity Lead to {FeNO} x and Significant Rate Enhancements in NO2- Reduction.J Am Chem Soc. 2018 Dec 12;140(49):17040-17050. doi: 10.1021/jacs.8b08520. Epub 2018 Nov 30. J Am Chem Soc. 2018. PMID: 30427681 Free PMC article.
References
-
- Moncada S., Palmer R. M. J., Higgs E. A. Pharmacol. Rev. 1991;43:109. - PubMed
- Ignarro L. J., in Nitric Oxide: Principles and Actions, ed. J. Lancaster Jr, Academic Press, New York, 1996, p. 111.
- Tennyson A. G., Lippard S. J. Chem. Biol. 2011;18:1211. - PubMed
- Kuhn V., Diederich L., Keller IV T. C. S., Kramer C. M., Lückstädt W., Panknin C., Suvorava T., Isakson B. E., Kelm M., Cortese-Krott M. M. Antioxid. Redox Signaling. 2017;26:718. - PMC - PubMed
-
- Lehnert N., Berto T. C., Galinato M. G. I. and Goodrich L. E., in The Handbook of Porphyrin Science, ed. K. M. Kadish, K. M. Smith and R. Guilard, World Scientific, Singapore, 2011, p. 1.
-
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Proc. Natl. Acad. Sci. U. S. A. 1990;87:1620. - PMC - PubMed
- Pacher P., Beckman J. S., Liaudet L. Physiol. Rev. 2007;87:315. - PMC - PubMed
- Lim C. H., Dedon P. C., Deen W. M. Chem. Res. Toxicol. 2008;21:2134. - PMC - PubMed
- Prolo C., Álvarez M. N., Radi R. BioFactors. 2014;40:215. - PMC - PubMed
- Lok H. C., Sahni S., Jansson P. J., Kovacevic Z., Hawkins C. L., Richardson D. R. J. Biol. Chem. 2016;291:27042. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

