Cellular and molecular responses to acute cocaine treatment in neuronal-like N2a cells: potential mechanism for its resistance in cell death
- PMID: 30210816
- PMCID: PMC6133924
- DOI: 10.1038/s41420-018-0078-x
Cellular and molecular responses to acute cocaine treatment in neuronal-like N2a cells: potential mechanism for its resistance in cell death
Erratum in
-
Erratum: Publisher Correction: articles initially published in wrong volume.Cell Death Discov. 2019 Jul 10;5:116. doi: 10.1038/s41420-019-0186-2. eCollection 2019. Cell Death Discov. 2019. PMID: 31312525 Free PMC article.
Abstract
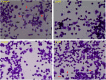
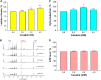
Cocaine is a highly abused drug that causes psychiatric and neurological problems. Its entry into neurons could alter cell-biochemistry and contribute in the manifestation of early pathological symptoms. We have previously shown the acute cocaine effects in rat C6 astroglia-like cells and found that these cells were highly sensitive to cocaine in terms of manifesting certain pathologies known to underlie psychological disorders. The present study was aimed to discern acute cocaine effects on the early onset of various changes in Neuro-2a (N2a) cells. Whole-cell patch-clamp recording of differentiated cells displayed the functional voltage-gated Na+ and K+ channels, which demonstrated the neuronal characteristics of the cells. Treatment of these cells with acute cocaine (1 h) at in vivo (nM to μM) and in vitro (mM) concentrations revealed that the cells remained almost 100% viable. Cocaine administration at 6.25 μM or 4 mM doses significantly reduced the inward currents but had no significant effect on outward currents, indicating the Na+ channel-blocking activity of cocaine. While no morphological change was observed at in vivo doses, treatment at in vitro doses altered the morphology, damaged the neurites, and induced cytoplasmic vacuoles; furthermore, general mitochondrial activity and membrane potential were significantly decreased. Mitochondrial dysfunction enabled the cells switch to anaerobic glycolysis, evidenced by dose-dependent increases in lactate and H2S, resulting unaltered ATP level in the cells. Further investigation on the mechanism of action unfolded that the cell's resistance to cocaine was through the activation of nuclear factor E2-related factor-2 (Nrf-2) gene and subsequent increase of antioxidants (glutathione [GSH], catalase and GSH peroxidase [GPx]). The data clearly indicate that the cells employed a detoxifying strategy against cocaine. On a broader perspective, we envision that extrapolating the knowledge of neuronal resistance to central nervous system (CNS) diseases could delay their onset or progression.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Haas C, Karila L, Lowenstein W. Cocaine and crack addiction: a growing public health problem. Bull. Acad. Natl. Med. 2009;193:947–962. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

