The Role of N-Acetylcysteine Supplementation on the Oxidative Stress Levels, Genotoxicity and Lineage Commitment Potential of Ex Vivo Murine Haematopoietic Stem/Progenitor Cells
- PMID: 30210840
- PMCID: PMC6132511
- DOI: 10.18295/squmj.2018.18.02.002
The Role of N-Acetylcysteine Supplementation on the Oxidative Stress Levels, Genotoxicity and Lineage Commitment Potential of Ex Vivo Murine Haematopoietic Stem/Progenitor Cells
Abstract
Objectives: The ex vivo maintenance of haematopoietic stem/progenitor cells (HSPCs) is crucial to ensure a sufficient supply of functional cells for research or therapeutic applications. However, when exposed to reactive oxygen species (ROS) in a normoxic microenvironment, HSPCs exhibit genomic instability which may diminish their quantity and quality. This study aimed to investigate the role of N-acetylcysteine (NAC) supplementation on the oxidative stress levels, genotoxicity and lineage commitment potential of murine haematopoietic stem/progenitor cells (HSPCs).
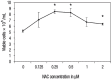
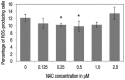
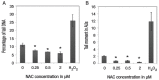
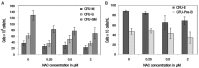
Methods: This study was carried out at the Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia, between June 2016 and July 2017. Bone marrow cells were isolated from nine mice and cultured in a growth medium. Various concentrations of NAC between 0.125-2 μM were added to the culture for 48 hours; these cells were then compared to non-supplemented cells harvested from the remaining three mice as the control group. A trypan blue exclusion test was performed to determine cell viability, while intracellular ROS levels and genotoxicity were determined by hydroethidine staining and comet assay, respectively. The lineage commitment potential of erythroid, myeloid and pre-B-lymphoid progenitor cells was evaluated via colony-forming cell assay.
Results: NAC supplementation at 0.25, 0.5 and 2 μM significantly increased cell viability (P <0.050), while intracellular ROS levels significantly decreased at 0.25 and 0.5 μM (P <0.050). Moreover, DNA damage was significantly reduced at all NAC concentrations (P <0.050). Finally, the potential lineage commitment of the cells was not significantly affected by NAC supplementation (P >0.050).
Conclusion: The findings of this study indicate that NAC supplementation may potentially overcome the therapeutic limitations of ex vivo-maintained HSPCs.
Keywords: Cell Lineage; DNA Damage; Hematopoietic Stem Cells; N-acetylcysteine; Reactive Oxygen Species.
Conflict of interest statement
CONFLICT OF INTEREST The authors declare no conflicts of interest.
Figures
Similar articles
-
The redox-sensitive transcription factor Nrf2 regulates murine hematopoietic stem cell survival independently of ROS levels.Blood. 2011 Dec 15;118(25):6572-9. doi: 10.1182/blood-2011-05-355362. Epub 2011 Oct 28. Blood. 2011. PMID: 22039262 Free PMC article.
-
Aging-Related Reduced Expression of CXCR4 on Bone Marrow Mesenchymal Stromal Cells Contributes to Hematopoietic Stem and Progenitor Cell Defects.Stem Cell Rev Rep. 2020 Aug;16(4):684-692. doi: 10.1007/s12015-020-09974-9. Stem Cell Rev Rep. 2020. PMID: 32418119 Free PMC article.
-
Lineage-related and particle size-dependent cytotoxicity of chitosan nanoparticles on mouse bone marrow-derived hematopoietic stem and progenitor cells.Food Chem Toxicol. 2015 Nov;85:31-44. doi: 10.1016/j.fct.2015.05.017. Epub 2015 Jun 5. Food Chem Toxicol. 2015. PMID: 26051352
-
Bone Marrow Oxidative Stress and Acquired Lineage-Specific Genotoxicity in Hematopoietic Stem/Progenitor Cells Exposed to 1,4-Benzoquinone.Int J Environ Res Public Health. 2020 Aug 13;17(16):5865. doi: 10.3390/ijerph17165865. Int J Environ Res Public Health. 2020. PMID: 32823552 Free PMC article.
-
The role of N-acetylcysteine in osteogenic microenvironment for bone tissue engineering.Front Cell Dev Biol. 2024 Jul 11;12:1435125. doi: 10.3389/fcell.2024.1435125. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39055649 Free PMC article. Review.
Cited by
-
Hepatocellular-Carcinoma-Derived Organoids: Innovation in Cancer Research.Cells. 2024 Oct 18;13(20):1726. doi: 10.3390/cells13201726. Cells. 2024. PMID: 39451244 Free PMC article. Review.
-
Clastogenicity and Aneugenicity of 1,4-Benzoquinone in Different Lineages of Mouse Hematopoietic Stem/Progenitor Cells.Toxics. 2021 May 12;9(5):107. doi: 10.3390/toxics9050107. Toxics. 2021. PMID: 34065823 Free PMC article.
-
Assessing the effects of N-acetyl cysteine on growth, antioxidant and immune response in tilapia (Oreochromis niloticus) under different regimes of stocking densities.PLoS One. 2024 Sep 30;19(9):e0307212. doi: 10.1371/journal.pone.0307212. eCollection 2024. PLoS One. 2024. PMID: 39348347 Free PMC article.
-
Ameliorative effects of hesperidin and N-acetylcysteine against formaldehyde-induced-hemato- and genotoxicity.Toxicol Res (Camb). 2021 Aug 24;10(5):992-1002. doi: 10.1093/toxres/tfab083. eCollection 2021 Oct. Toxicol Res (Camb). 2021. PMID: 34733484 Free PMC article.
-
Radiation-induced bystander effects impair transplanted human hematopoietic stem cells via oxidative DNA damage.Blood. 2021 Jun 17;137(24):3339-3350. doi: 10.1182/blood.2020007362. Blood. 2021. PMID: 33881475 Free PMC article.
References
-
- Ludin A, Gur-Cohen S, Golan K, Kaufmann KB, Itkin T, Medaglia C, et al. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid Redox Signal. 2014;21:1605–19. doi: 10.1089/ars.2014.5941. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
