Ammonium Ylide Mediated Cyclization Reactions
- PMID: 30210965
- PMCID: PMC6130843
- DOI: 10.1002/ajoc.201800091
Ammonium Ylide Mediated Cyclization Reactions
Abstract
The use of readily accessible ammonium ylides for (asymmetric) transformations, especially cyclization reactions, has received considerable attention over the past two decades. A variety of highly enantioselective protocols to facilitate annulation reactions have recently been introduced as an alternative to other common methods including S-ylide-mediated strategies. It is the intention of this short review to provide an introduction to this field by highlighting the potential of ammonium ylides for (asymmetric) cyclization reactions as well as to present the limitations and challenges of these methods.
Keywords: asymmetric synthesis; cyclization; heterocycles; small ring systems; ylides.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest.
Figures











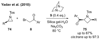


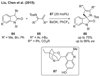

References
-
-
For the first report that describes an ylide, see: Michaelis A, Gimborn HV. Ber Dtsch Chem Ges. 1894;27:272–277. doi: 10.1002/cber.18940270152.
-
-
- Li A-H, Dai L-X, Aggarwal VK. Chem Rev. 1997;97:2341–2372. doi: 10.1021/cr960411r. - DOI - PubMed
- McGarrigle EM, Aggarwal VK. In: Enantioselective Organocatalysis. Dalko P, editor. Wiley-VCH; Weinheim: 2007. pp. 357–389.
- Wentrup C. Acc Chem Res. 2011;44:393–404. doi: 10.1021/ar700198z. - DOI - PubMed
- Liao S, Wang P, Tang Y. In: Comprehensive Enantioselective Organocatalysis. Dalko P, editor. Wiley-VCH; Weinheim: 2013. pp. 547–577.
- Clark JS, editor. Nitrogen, Oxygen and Sulfur Ylide Chemistry. Oxford University Press; Oxford: 2002.
-
- Moss GP, Smith PAS, Taverner D. Pure Appl Chem. 1995;67:1307–1375.
-
-
For recent reviews on P-ylides, see: Byrne PA, Gilheany DG. Chem Soc Rev. 2013;42:6670–6696. doi: 10.1039/C3CS60105F. Zhou R, He Z. Eur J Org Chem. 2016:1937–1954. doi: 10.1002/ejoc.201501585. Selva M, Perosa A, Noe M. In: Organophosphorus Chemistry. Allen DW, Loakes D, Tebby JC, editors. Vol. 44. RSC; Cambridge: 2015. pp. 136–169.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
