Comprehensive genotype-phenotype correlation in lissencephaly
- PMID: 30211035
- PMCID: PMC6127521
- DOI: 10.21037/qims.2018.08.08
Comprehensive genotype-phenotype correlation in lissencephaly
Abstract
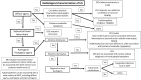
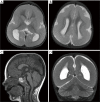
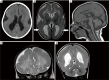
Malformations of cortical development (MCD) are a heterogenous group of disorders with diverse genotypic and phenotypic variations. Lissencephaly is a subtype of MCD caused by defect in neuronal migration, which occurs between 12 and 24 weeks of gestation. The continuous advancement in the field of molecular genetics in the last decade has led to identification of at least 19 lissencephaly-related genes, most of which are related to microtubule structural proteins (tubulin) or microtubule-associated proteins (MAPs). The aim of this review article is to bring together current knowledge of gene mutations associated with lissencephaly and to provide a comprehensive genotype-phenotype correlation. Illustrative cases will be presented to facilitate the understanding of the described genotype-phenotype correlation.
Keywords: LIS1; Lissencephaly (LIS); doublecortin (DCX); tubulinopathy.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures

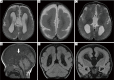
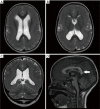
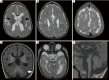
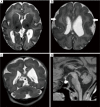

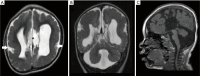
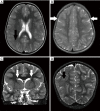
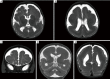
References
-
- des Portes V, Francis F, Pinard JM, Desguerre I, Moutard ML, Snoeck I, Meiners LC, Capron F, Cusmai R, Ricci S, Motte J, Echenne B, Ponsot G, Dulac O, Chelly J, Beldjord C. Doublecortin is the major gene causing X-linked subcortical laminar heterotopia (SCLH). Hum Mol Genet 1998;7:1063-70. 10.1093/hmg/7.7.1063 - DOI - PubMed
-
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell 1998;92:63-72. 10.1016/S0092-8674(00)80899-5 - DOI - PubMed
-
- Abdollahi MR, Morrison E, Sirey T, Molnar Z, Hayward BE, Carr IM, Springell K, Woods CG, Ahmed M, Hattingh L, Corry P, Pilz DT, Stoodley N, Crow Y, Taylor GR, Bonthron DT, Sheridan E. Mutation of the variant alpha-tubulin TUBA8 results in polymicrogyria with optic nerve hypoplasia. Am J Hum Genet 2009;85:737-44. 10.1016/j.ajhg.2009.10.007 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
