Paclitaxel Plus Cetuximab as 1st Line Chemotherapy in Platinum-Based Chemoradiotherapy-Refractory Patients With Squamous Cell Carcinoma of the Head and Neck
- PMID: 30211118
- PMCID: PMC6119881
- DOI: 10.3389/fonc.2018.00339
Paclitaxel Plus Cetuximab as 1st Line Chemotherapy in Platinum-Based Chemoradiotherapy-Refractory Patients With Squamous Cell Carcinoma of the Head and Neck
Abstract
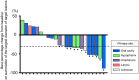
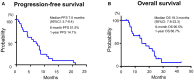
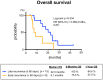
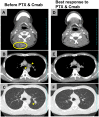
Purpose: We sought to evaluate the efficacy and safety of the combination of cetuximab (Cmab) and paclitaxel (PTX) in patients with squamous cell carcinoma of the head and neck (SCCHN) who had unresectable recurrent or metastatic (R/M) disease after platinum-based chemoradiotherapy. Materials and Methods: Data on 23 patients with SCCHN who received paclitaxel and cetuximab (Cmab) for R/M disease no more than 6 months after CRT completion were retrospectively reviewed. PTX and Cmab were given in a 28-day cycle (PTX, 80 mg/m2 on days 1, 8, and 15; Cmab, loading dose 400 mg/m2 followed by a weekly 250 mg/m2). The differences in prognosis between subgroups in different clinical settings were also assessed. Results: CRT had been delivered as definitive treatment in 13 cases (57%) and as adjuvant treatment in 10 (43%). Median time from CRT completion to disease recurrence or metastasis was 73 days (1-152). The best objective response and disease control rates were 52 and 83%, respectively, with 12 partial responses and seven cases of stable disease by Response Evaluation Criteria in Solid Tumors (RECIST). A total of 17 of 23 patients (74%) achieved a degree of tumor shrinkage. Median progression-free survival (PFS) and overall survival (OS) were 7.0 (95% confidence interval [CI]: 3.7-8.4) and 16.3 months (95% CI: 7.8-23.3), respectively. Patients with a longer duration (≥60 d) from CRT completion to disease progression had a statistically significantly longer OS than the others (median OS 22.3 vs. 8.1 months, log-rank test; p = 0.034). Main Grade 3 toxicities included neutropenia (13%), anemia (13%), and hypomagnesemia (13%). No Grade 4 toxicity or treatment-related death was seen. Conclusion: PTX and Cmab is a tolerable and effective option in SCCHN patients with symptomatic CRT-refractory disease. Its favorable effects on tumor shrinkage will help relieve tumor-associated symptoms.
Keywords: cetuximab; chemoradiotherapy; paclitaxel; platinum-refractory; squamous cell carcinoma of the head and neck.
Figures
Similar articles
-
A multicenter phase II trial of paclitaxel, carboplatin, and cetuximab followed by chemoradiotherapy in patients with unresectable locally advanced squamous cell carcinoma of the head and neck.Cancer Med. 2020 Mar;9(5):1671-1682. doi: 10.1002/cam4.2852. Epub 2020 Jan 13. Cancer Med. 2020. PMID: 31943834 Free PMC article. Clinical Trial.
-
Clinical impact of weekly paclitaxel plus cetuximab is comparable to the EXTREME regimen for recurrent/metastatic head and neck squamous cell carcinoma.Int J Clin Oncol. 2021 Jul;26(7):1188-1195. doi: 10.1007/s10147-021-01907-x. Epub 2021 Apr 5. Int J Clin Oncol. 2021. PMID: 33821363
-
Efficacy and safety of weekly paclitaxel combined with cetuximab in the treatment of pretreated recurrent/metastatic head and neck cancer patients.Oral Oncol. 2013 Feb;49(2):182-5. doi: 10.1016/j.oraloncology.2012.09.003. Epub 2012 Sep 28. Oral Oncol. 2013. PMID: 23026069
-
Clinical outcomes with therapies for previously treated recurrent/metastatic head-and-neck squamous cell carcinoma (R/M HNSCC): A systematic literature review.Oral Oncol. 2018 Sep;84:108-120. doi: 10.1016/j.oraloncology.2018.07.005. Epub 2018 Aug 1. Oral Oncol. 2018. PMID: 30115469
-
Afatinib in squamous cell carcinoma of the head and neck.Expert Opin Pharmacother. 2016 Jun;17(9):1295-301. doi: 10.1080/14656566.2016.1183647. Epub 2016 May 19. Expert Opin Pharmacother. 2016. PMID: 27160335 Review.
Cited by
-
Immune Checkpoint Inhibitors for Nasopharyngeal Carcinoma in a Real-world Setting in Japan.In Vivo. 2023 Mar-Apr;37(2):747-755. doi: 10.21873/invivo.13137. In Vivo. 2023. PMID: 36881083 Free PMC article.
-
Real-world Data of Paclitaxel and Cetuximab in Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck.Cancer Diagn Progn. 2023 Mar 3;3(2):264-271. doi: 10.21873/cdp.10211. eCollection 2023 Mar-Apr. Cancer Diagn Progn. 2023. PMID: 36875311 Free PMC article.
-
Effectiveness and safety of weekly paclitaxel and cetuximab as a salvage chemotherapy following immune checkpoint inhibitors for recurrent or metastatic head and neck squamous cell carcinoma: A multicenter clinical study.PLoS One. 2022 Jul 28;17(7):e0271907. doi: 10.1371/journal.pone.0271907. eCollection 2022. PLoS One. 2022. PMID: 35901098 Free PMC article.
-
Cetuximab combined with paclitaxel or paclitaxel alone for patients with recurrent or metastatic head and neck squamous cell carcinoma progressing after EXTREME.Cancer Med. 2021 Jun;10(12):3952-3963. doi: 10.1002/cam4.3953. Epub 2021 May 25. Cancer Med. 2021. PMID: 34080776 Free PMC article.
-
Efficacy and Safety of Paclitaxel Combined With Cetuximab for Head and Neck Squamous Cell Carcinoma.In Vivo. 2021 Mar-Apr;35(2):1253-1259. doi: 10.21873/invivo.12376. In Vivo. 2021. PMID: 33622928 Free PMC article.
References
-
- Stewart BW, Kleihues P. International Agency for Research on Cancer Press. World cancer report. Lyon: (2003).
-
- Adelstein DJ, Li Y, Adams GL, Wanger H Jr, Kish JA, Enslery JF, et al. . An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. (2003) 21:92–8. 10.1200/JCO.2003.01.008 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

