Polyethylene Oxide and Silicon-Substituted Hydroxyapatite Composite: A Biomaterial for Hard Tissue Engineering in Orthopedic and Spine Surgery
- PMID: 30211130
- PMCID: PMC6124219
- DOI: 10.4103/abr.abr_206_17
Polyethylene Oxide and Silicon-Substituted Hydroxyapatite Composite: A Biomaterial for Hard Tissue Engineering in Orthopedic and Spine Surgery
Abstract
Background: Tissue engineering and biomaterials have made it possible to innovate bone treatments for orthopedic and spine problems. The aim of this study is to develop a novel polyethylene oxide (PEO)/silicon-substituted hydroxyapatite (Si-HA) composite to be used as a scaffold for hard tissue engineering in orthopedic and spine procedures.
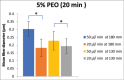
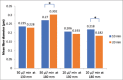
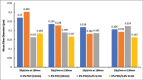
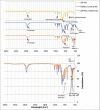
Materials and methods: The composite was fabricated through the electrospinning technique. The applied voltage (5 kV) and PEO concentration (5%) were fixed. Processing parameters such as the flow rates (20 μl/min and 50 μl/min), distances from capillary tube to the collector (130 mm and 180 mm), spinning time (10 min and 20 min), and concentration of Si-HA (0.2% and 0.6%) were explored to find the optimum conditions to produce fine composite fibers.
Results: Scanning electron microscope images showed that 5% PEO, 5% PEO/0.2% Si-HA, and 5% PEO/0.6% Si-HA fibers were successively produced. Flow rates and working distances showed significant influence on the morphology of the polymeric and composite fibers. A high flow rate (50 μl/min) and a larger working distance (180 mm) resulted in larger fibers. The comparison between the mean fiber diameter of 5% PEO/0.2% Si-HA and 5% PEO/0.6% Si-HA showed to be significantly different. As the Si-HA concentration increased, certain fibers were having particles of Si-HA that were not properly integrated into the polymer matrix.
Conclusions: Synthesis of a novel biomaterial for hard tissue scaffold through electrospinning was successful. In general, PEO/Si-HA fibers produced have the desired characteristics to mimic the extracellular matrix of bone.
Keywords: Biomaterial; hard tissue engineering; neurosurgery; orthopedics; polyethylene oxide; silicon-substituted hydroxyapatite; spine surgery.
Conflict of interest statement
There are no conflicts of interest.
Figures





Similar articles
-
Optimization of electrospinning process & parameters for producing defect-free chitosan/polyethylene oxide nanofibers for bone tissue engineering.J Biomater Sci Polym Ed. 2020 Apr;31(6):781-803. doi: 10.1080/09205063.2020.1718824. Epub 2020 Jan 29. J Biomater Sci Polym Ed. 2020. PMID: 31958253
-
Preparation and biological properties of ZnO/hydroxyapatite/chitosan-polyethylene oxide@gelatin biomimetic composite scaffolds for bone tissue engineering.J Biomater Appl. 2022 Aug;37(2):238-248. doi: 10.1177/08853282221087110. Epub 2022 Apr 29. J Biomater Appl. 2022. PMID: 35487772
-
Silicon-incorporated nanohydroxyapatite-reinforced poly(ε-caprolactone) film to enhance osteogenesis for bone tissue engineering applications.Colloids Surf B Biointerfaces. 2020 Mar;187:110714. doi: 10.1016/j.colsurfb.2019.110714. Epub 2019 Dec 9. Colloids Surf B Biointerfaces. 2020. PMID: 31870518
-
The Use of Fibers in Bone Tissue Engineering.Tissue Eng Part B Rev. 2022 Feb;28(1):141-159. doi: 10.1089/ten.TEB.2020.0252. Epub 2021 Feb 17. Tissue Eng Part B Rev. 2022. PMID: 33375900 Review.
-
Nanoscale characterization of the interface between bone and hydroxyapatite implants and the effect of silicon on bone apposition.Micron. 2006;37(8):681-8. doi: 10.1016/j.micron.2006.03.006. Epub 2006 Mar 31. Micron. 2006. PMID: 16632368 Review.
Cited by
-
Design and Fabrication of Sustained Bacterial Release Scaffolds to Support the Microbiome.Pharmaceutics. 2024 Aug 14;16(8):1066. doi: 10.3390/pharmaceutics16081066. Pharmaceutics. 2024. PMID: 39204410 Free PMC article.
References
-
- National Osteoporosis Society. A Strategy to Reduce the Impact of Osteoporosis and Fragility Fractures in England. 2009. [Last accessed on 2018 Jun 20]. Available from: http://www.nos.org.uk/document.doc?id=491 .
-
- Cooper C, Campion G, Melton LJ., 3rd Hip fractures in the elderly: A world-wide projection. Osteoporos Int. 1992;2:285–9. - PubMed
-
- Huiskes R, Weinans H, van Rietbergen B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop Relat Res. 1992;274:124–34. - PubMed
-
- Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295:1014–7. - PubMed
-
- Archibeck MJ, Berger RA, Jacobs JJ, Quigley LR, Gitelis S, Rosenberg AG, et al. Second-generation cementless total hip arthroplasty. Eight to eleven-year results. J Bone Joint Surg Am. 2001;83-A:1666–73. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

