A Five-Component Biginelli-Diels-Alder Cascade Reaction
- PMID: 30211156
- PMCID: PMC6119773
- DOI: 10.3389/fchem.2018.00376
A Five-Component Biginelli-Diels-Alder Cascade Reaction
Abstract
A new multi-component condensation was discovered during the reaction of a urea, β-keto ester, and formaldehyde. In the presence of catalytic indium bromide, a Biginelli dihydropyrimidinone intermediate was further converted to a five-component condensation product through a formal hetero Diels-Alder reaction. The product structure was confirmed by NMR and NOE analysis, and the proposed stepwise mechanism was supported by the reaction of the Biginelli intermediate with ethyl 2-methylene-3-oxobutanoate.
Keywords: 5-CC; Biginelli reaction; InBr3; dihydropyrimidinone; hetero Diels-Alder reaction; multi-component condensation.
Figures



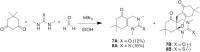

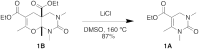

References
-
- Fu N. Y., Yuan Y. F., Cao Z., Wang S. W., Wang J. T., Peppe C. (2002). Indium(III) bromide-catalyzed preparation of dihydropyrimidinones: improved protocol conditions for the Biginelli reaction. Tetrahedron 58, 4801–4807. 10.1016/S0040-4020(02)00455-6 - DOI
-
- Hu E. H., Sidler D. R., Dolling U.-H. (1998). Unprecedented catalytic three component one-pot condensation reaction:an efficient synthesis of 5-alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones. J. Organ. Chem. 63, 3454–3457. 10.1021/jo970846u - DOI
-
- Huryn D. M., Brodsky J. L., Brummond K. M., Chambers P. G., Eyer B., Ireland A. W., et al. . (2011). Chemical methodology as a source of small-molecule checkpoint inhibitors and heat shock protein 70 (Hsp70) modulators. Proc. Natl. Acad. Sci. U.S.A. 108, 6757–6762. 10.1073/pnas.1015251108 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

