On the Way to in vitro Platelet Production
- PMID: 30211166
- PMCID: PMC6120994
- DOI: 10.3389/fmed.2018.00239
On the Way to in vitro Platelet Production
Abstract
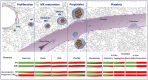
The severely decreased platelet counts (10-30. 103 platelets/μL) frequently observed in patients undergoing chemotherapy, radiation treatment, or organ transplantation are associated with life-threatening increased bleeding risks. To circumvent these risks, platelet transfusion remains the treatment of choice, despite some limitations which include a limited shelf-life, storage-related deterioration, the development of alloantibodies in recipients and the transmission of infectious diseases. A sustained demand has evolved in recent years for controlled blood products, free of infectious, inflammatory, and immune risks. As a consequence, the challenge for blood centers in the near future will be to ensure an adequate supply of blood platelets, which calls for a reassessment of our transfusion models. To meet this challenge, many laboratories are now turning their research efforts toward the in vitro and customized production of blood platelets. In recent years, there has been a major enthusiasm for the cultured platelet production, as illustrated by the number of reviews that have appeared in recent years. The focus of the present review is to critically asses the arguments put forward in support of the culture of platelets for transfusion purposes. In light of this, we will recapitulate the main advances in this quickly evolving field, while noting the technical limitations to overcome to make cultured platelet a transfusional alternative.
Keywords: biomanufacturing; hematopoietic stem cells; in vitro production; megakaryocytes; platelets.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

