An Improved Inhaled Mucolytic to Treat Airway Muco-obstructive Diseases
- PMID: 30212240
- PMCID: PMC6353008
- DOI: 10.1164/rccm.201802-0245OC
An Improved Inhaled Mucolytic to Treat Airway Muco-obstructive Diseases
Abstract
Rationale: Airways obstruction with thick, adherent mucus is a pathophysiologic and clinical feature of muco-obstructive respiratory diseases, including chronic obstructive pulmonary disease, asthma, and cystic fibrosis (CF). Mucins, the dominant biopolymer in mucus, organize into complex polymeric networks via the formation of covalent disulfide bonds, which govern the viscoelastic properties of the mucus gel. For decades, inhaled N-acetylcysteine (NAC) has been used as a mucolytic to reduce mucin disulfide bonds with little, if any, therapeutic effects. Improvement of mucolytic therapy requires the identification of NAC deficiencies and the development of compounds that overcome them.
Objectives: Elucidate the pharmacological limitations of NAC and test a novel mucin-reducing agent, P3001, in preclinical settings.
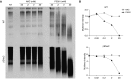
Methods: The study used biochemical (e.g., Western blotting, mass spectrometry) and biophysical assays (e.g., microrheology/macrorheology, spinnability, mucus velocity measurements) to test compound efficacy and toxicity in in vitro and in vivo models and patient sputa.
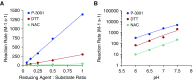
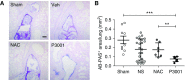
Measurements and main results: Dithiothreitol and P3001 were directly compared with NAC in vitro and both exhibited superior reducing activities. In vivo, P3001 significantly decreased lung mucus burden in βENaC-overexpressing mice, whereas NAC did not (n = 6-24 mice per group). In NAC-treated CF subjects (n = 5), aerosolized NAC was rapidly cleared from the lungs and did not alter sputum biophysical properties. In contrast, P3001 acted faster and at lower concentrations than did NAC, and it was more effective than DNase in CF sputum ex vivo.
Conclusions: These results suggest that reducing the viscoelasticity of airway mucus is an achievable therapeutic goal with P3001 class mucolytic agents.
Keywords: mucins; mucociliary clearance; mucus; obstructive pulmonary diseases; reducing agents.
Figures





Comment in
-
Dawn of a New Era in the Diagnosis and Treatment of Airway Mucus Dysfunction.Am J Respir Crit Care Med. 2019 Jan 15;199(2):133-134. doi: 10.1164/rccm.201808-1444ED. Am J Respir Crit Care Med. 2019. PMID: 30252497 No abstract available.
References
-
- Perez-Vilar J, Boucher RC. Reevaluating gel-forming mucins’ roles in cystic fibrosis lung disease. Free Radic Biol Med. 2004;37:1564–1577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

