Integrated Treatment for Smoking Cessation, Anxiety, and Depressed Mood in People Living With HIV: A Randomized Controlled Trial
- PMID: 30212438
- PMCID: PMC10041790
- DOI: 10.1097/QAI.0000000000001787
Integrated Treatment for Smoking Cessation, Anxiety, and Depressed Mood in People Living With HIV: A Randomized Controlled Trial
Abstract
Objective: Among people living with HIV, cigarette smoking rates are higher than among the general population, and anxiety, depression, and their disorders are common and associated with smoking and poorer outcomes during cessation. This study evaluated the efficacy of an integrated smoking cessation intervention, developed to target anxiety, depression, and smoking cessation concurrently among people living with HIV.
Method: Smokers living with HIV who reported at least moderate motivation to quit smoking were randomized into a novel 9-week integrated intervention (QUIT), consisting of 1 psychoeducation (prerandomization) session and 9 weekly 1-hour sessions of cognitive behavioral therapy for smoking cessation and anxiety/depression plus nicotine replacement therapy, or a 9-week enhanced standard smoking intervention (ETAU), consisting of 1 psychoeducation session (prerandomization) and 4 brief weekly check-in sessions plus nicotine replacement therapy. All were instructed to make a quit attempt at week 6.
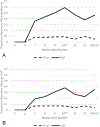
Results: Seventy-two participants were enrolled, and 53 were randomized. 41/53 participants completed the active treatment phase of the study. 7-day point-prevalence abstinence, verified with expired carbon monoxide, was significantly higher among those in the integrated intervention than those in the enhanced standard intervention both end-of-treatment {[MQUIT = 59%, METAU = 9%; b = 5.60, 95% confidence interval: (2.64 to 8.56), t(332) = 3.72, P < 0.001]} and 6-months post-quit date {[MQUIT = 46%, METAU = 5%; b = 7.69, 95% confidence interval: (4.60 to 10.78), t(332) = 4.90, P < 0.001]}. Consideration of patterns of missingness did not alter the significance of these findings.
Conclusions: The integrated intervention was associated with substantially higher short-term and long-term abstinence rates than the enhanced standard intervention. These data provide promising initial evidence supporting the benefits of an integrated anxiety-depression/smoking cessation program specifically tailored for people living with HIV.
Trial registration: ClinicalTrials.gov NCT01393301.
Conflict of interest statement
J.A.J.S. has received compensation from Microtransponder, Inc., and Aptinyx, Inc. for consulting. The remaining authors have no conflicts of interests to disclose.
Figures


References
-
- U.S. Department of Health and Human Services. The health consequences of smoking—50 years of progress. A report of the surgeon general. In: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. Atlanta, GA: Department of Health and Human Services; 2014.
-
- Niaura R, Shadel W, Morrow K, et al. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin Infect Dis. 2000;3:808–812. - PubMed
-
- Mdodo R, Frazier E, Dube S, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162:335–344. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

