Performance and usability evaluation of the INSTI HIV self-test in Kenya for qualitative detection of antibodies to HIV
- PMID: 30212525
- PMCID: PMC6136890
- DOI: 10.1371/journal.pone.0202491
Performance and usability evaluation of the INSTI HIV self-test in Kenya for qualitative detection of antibodies to HIV
Abstract
Background: HIV testing is often undermined by lack of confidentiality, stigma, shortage of counselors and long distances to testing centers. Self-testing has the potential to circumvent these constraints.
Objective: To determine the performance and usability characteristics of the INSTI® HIV-1/HIV-2 Self-Test.
Methods: The performance evaluation was a cross sectional study and the usability a mixed methods study. For method comparison, Bioelisa HIV-1+2 Ag/Ab test was used as the reference test. When the test results were discrepant, results from Alere Determine™ HIV-1/2 and First Response HIV-1-2 Antibody tests were used for confirmation of status.
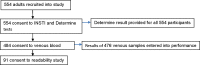
Results: Sensitivity of the INSTI HIV Self-Test was 98.99% (95% CI 96.05-99.75%), and specificity 98.15% (95% CI 95.63-99.23%). The concordance was therefore 97.27%. A total of 354 participants took part in the usability study. Of those, 343 (98.00%) found instructions for use easy to follow, 330 (94.29%) found the finger prick device easy to use, 303 (86.57%) were confident while performing the test, 342 (97.71%) felt result interpretation was easy, while 304 (86.86%) declared results within the recommended five minutes. Three hundred and forty two (342, 97.71%) were willing to use the test again while 344 (98.29%) would recommend the kit to a sexual partner. None of the 350 participants quit the process at any stage. Three hundred and eighteen (318, 91.12%) participants felt the test needed no further improvement. All 91 lay users correctly identified cartridges that showed positive, negative and invalid results. Only 31 (34.07%) participants correctly identified weak positive dummy test results.
Conclusion: The excellent performance and usability characteristics of INSTI HIV-1/HIV-2 self-test make the kit a viable option for HIV self-testing. To improve the identification of weak positive results, the manufacturer should indicate on the IFU that even a faint test spot should be interpreted as positive.
Conflict of interest statement
bioLytical Inc. (Richmond, BC, Canada) provided the test cartridges for this study. None of the authors have held any employment, consultancy, patents, or commercial interest in products in development, or marketed products from bioLytical Inc. Support from bioLytical Inc. does not alter the authors’ adherence to all PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
A prospective multicentre study of healthcare provider preference in rapid HIV testing kits: Determine versus INSTI.Int J STD AIDS. 2018 Jan;29(1):51-56. doi: 10.1177/0956462417717648. Epub 2017 Jul 1. Int J STD AIDS. 2018. PMID: 28669324
-
What should the ideal HIV self-test look like? A usability study of test prototypes in unsupervised HIV self-testing in Kenya, Malawi, and South Africa.AIDS Behav. 2014 Jul;18 Suppl 4:S422-32. doi: 10.1007/s10461-014-0818-8. AIDS Behav. 2014. PMID: 24947852
-
Accuracy and user-acceptability of HIV self-testing using an oral fluid-based HIV rapid test.PLoS One. 2012;7(9):e45168. doi: 10.1371/journal.pone.0045168. Epub 2012 Sep 17. PLoS One. 2012. PMID: 23028822 Free PMC article.
-
[Screening tests combined with p24 antigen and anti-HIV antibodies in early detection of HIV-1].Transfus Clin Biol. 2000 Jun;7 Suppl 1:18s-24s. doi: 10.1016/s1246-7820(00)80011-7. Transfus Clin Biol. 2000. PMID: 10919219 Review. French.
-
The INSTI HIV-1/HIV-2 antibody test: a review.Expert Opin Med Diagn. 2013 May;7(3):299-308. doi: 10.1517/17530059.2013.774370. Epub 2013 Feb 25. Expert Opin Med Diagn. 2013. PMID: 23480561 Review.
Cited by
-
Impact of HIV self-testing for oral pre-exposure prophylaxis scale-up on drug resistance and HIV outcomes in western Kenya: a modelling study.Lancet HIV. 2024 Mar;11(3):e167-e175. doi: 10.1016/S2352-3018(23)00268-0. Epub 2024 Jan 29. Lancet HIV. 2024. PMID: 38301668 Free PMC article.
-
A Lay-User Assessment of Hepatitis C Virus Self-Testing Device Usability and Interpretation in Johannesburg, South Africa.Diagnostics (Basel). 2021 Mar 7;11(3):463. doi: 10.3390/diagnostics11030463. Diagnostics (Basel). 2021. PMID: 33800060 Free PMC article.
-
Implementation outcomes of HIV self-testing in low- and middle- income countries: A scoping review.PLoS One. 2021 May 3;16(5):e0250434. doi: 10.1371/journal.pone.0250434. eCollection 2021. PLoS One. 2021. PMID: 33939722 Free PMC article.
-
Acceptability, feasibility, and accuracy of blood-based HIV self-testing: A cross-sectional study in Ho Chi Minh City, Vietnam.PLOS Glob Public Health. 2023 Feb 1;3(2):e0001438. doi: 10.1371/journal.pgph.0001438. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 36962976 Free PMC article.
-
Findings from a novel and scalable community-based HIV testing approach to reduce the time required to complete point-of-care HIV testing in South Africa.BMC Health Serv Res. 2021 Oct 29;21(1):1176. doi: 10.1186/s12913-021-07173-x. BMC Health Serv Res. 2021. PMID: 34711236 Free PMC article.
References
-
- Government of Kenya. Kenya Demographic and Health Survey 2014:Key Indicators Report. 2015 2015.
-
- National AIDS and STI Control Programme (NASCOP) K. Kenya AIDS Indicator Survey 2012: Final Report. 2014. 2014.
-
- National AIDS and STI control programme (NASCOP). National Guidelines for HIV Testing and Counselling in Kenya. 2008.
-
- UNAIDS. Prevention Gap Report. 2016.
-
- National AIDS and STI control programme (NASCOP). National Guidelines for HIV Testing and Counselling in Kenya. 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical