Performance and usability evaluation of the INSTI HIV self-test in Kenya for qualitative detection of antibodies to HIV
- PMID: 30212525
- PMCID: PMC6136890
- DOI: 10.1371/journal.pone.0202491
Performance and usability evaluation of the INSTI HIV self-test in Kenya for qualitative detection of antibodies to HIV
Abstract
Background: HIV testing is often undermined by lack of confidentiality, stigma, shortage of counselors and long distances to testing centers. Self-testing has the potential to circumvent these constraints.
Objective: To determine the performance and usability characteristics of the INSTI® HIV-1/HIV-2 Self-Test.
Methods: The performance evaluation was a cross sectional study and the usability a mixed methods study. For method comparison, Bioelisa HIV-1+2 Ag/Ab test was used as the reference test. When the test results were discrepant, results from Alere Determine™ HIV-1/2 and First Response HIV-1-2 Antibody tests were used for confirmation of status.
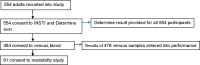
Results: Sensitivity of the INSTI HIV Self-Test was 98.99% (95% CI 96.05-99.75%), and specificity 98.15% (95% CI 95.63-99.23%). The concordance was therefore 97.27%. A total of 354 participants took part in the usability study. Of those, 343 (98.00%) found instructions for use easy to follow, 330 (94.29%) found the finger prick device easy to use, 303 (86.57%) were confident while performing the test, 342 (97.71%) felt result interpretation was easy, while 304 (86.86%) declared results within the recommended five minutes. Three hundred and forty two (342, 97.71%) were willing to use the test again while 344 (98.29%) would recommend the kit to a sexual partner. None of the 350 participants quit the process at any stage. Three hundred and eighteen (318, 91.12%) participants felt the test needed no further improvement. All 91 lay users correctly identified cartridges that showed positive, negative and invalid results. Only 31 (34.07%) participants correctly identified weak positive dummy test results.
Conclusion: The excellent performance and usability characteristics of INSTI HIV-1/HIV-2 self-test make the kit a viable option for HIV self-testing. To improve the identification of weak positive results, the manufacturer should indicate on the IFU that even a faint test spot should be interpreted as positive.
Conflict of interest statement
bioLytical Inc. (Richmond, BC, Canada) provided the test cartridges for this study. None of the authors have held any employment, consultancy, patents, or commercial interest in products in development, or marketed products from bioLytical Inc. Support from bioLytical Inc. does not alter the authors’ adherence to all PLOS ONE policies on sharing data and materials.
Figures
References
-
- Government of Kenya. Kenya Demographic and Health Survey 2014:Key Indicators Report. 2015 2015.
-
- National AIDS and STI Control Programme (NASCOP) K. Kenya AIDS Indicator Survey 2012: Final Report. 2014. 2014.
-
- National AIDS and STI control programme (NASCOP). National Guidelines for HIV Testing and Counselling in Kenya. 2008.
-
- UNAIDS. Prevention Gap Report. 2016.
-
- National AIDS and STI control programme (NASCOP). National Guidelines for HIV Testing and Counselling in Kenya. 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical