CD4+T cells mediate protection against Zika associated severe disease in a mouse model of infection
- PMID: 30212537
- PMCID: PMC6136803
- DOI: 10.1371/journal.ppat.1007237
CD4+T cells mediate protection against Zika associated severe disease in a mouse model of infection
Abstract
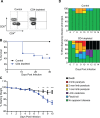
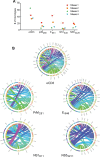
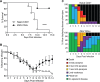
Zika virus (ZIKV) has gained worldwide attention since it emerged, and a global effort is underway to understand the correlates of protection and develop diagnostics to identify rates of infection. As new therapeutics and vaccine approaches are evaluated in clinical trials, additional effort is focused on identifying the adaptive immune correlates of protection against ZIKV disease. To aid in this endeavor we have begun to dissect the role of CD4+T cells in the protection against neuroinvasive ZIKV disease. We have identified an important role for CD4+T cells in protection, demonstrating that in the absence of CD4+T cells mice have more severe neurological sequela and significant increases in viral titers in the central nervous system (CNS). The transfer of CD4+T cells from ZIKV immune mice protect type I interferon receptor deficient animals from a lethal challenge; showing that the CD4+T cell response is necessary and sufficient for control of ZIKV disease. Using a peptide library spanning the complete ZIKV polyprotein, we identified both ZIKV-encoded CD4+T cell epitopes that initiate immune responses, and ZIKV specific CD4+T cell receptors that recognize these epitopes. Within the ZIKV antigen-specific TCRβ repertoire, we uncovered a high degree of diversity both in response to a single epitope and among different mice responding to a CD4+T cell epitope. Overall this study identifies a novel role for polyfunctional and polyclonal CD4+T cells in providing protection against ZIKV infection and highlights the need for vaccines to develop robust CD4+T cell responses to prevent ZIKV neuroinvasion and limit replication within the CNS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1952;46(5):509–20. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

