Biomarkers for diagnosis of childhood tuberculosis: A systematic review
- PMID: 30212540
- PMCID: PMC6136789
- DOI: 10.1371/journal.pone.0204029
Biomarkers for diagnosis of childhood tuberculosis: A systematic review
Abstract
Introduction: As studies of biomarkers of tuberculosis (TB) disease provide hope for a simple, point-of-care test, we aimed to synthesize evidence on biomarkers for diagnosis of TB in children and compare their accuracy to published target product profiles (TPP).
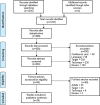
Methods: We conducted a systematic review of biomarkers for diagnosis of pulmonary TB in exclusively paediatric populations, defined as age less than 15 years. PubMed, EMBASE and Web of Science were searched for relevant publications from January 1, 2000 to November 27, 2017. Studies using mixed adult and paediatric populations or reporting biomarkers for extrapulmonary TB were excluded. Study quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) framework. No meta-analysis was done because the published childhood TB biomarkers studies were mostly early stage studies and highly heterogeneous.
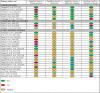
Results: The 29 studies included in this systematic review comprise 20 case-control studies, six cohort studies and three cross-sectional studies. These studies reported diverse and heterogeneous forms of biomarkers requiring different types of clinical specimen and laboratory assays. Majority of the studies (27/29 [93%]) either did not meet the criteria in at least one of the four domains of the QUADAS-2 reporting framework or the assessment was unclear. However, the diagnostic performance of biomarkers reported in 22 studies met one or both of the WHO-recommended minimal targets of 66% sensitivity and 98% specificity for a new diagnostic test for TB disease in children, and/or 90% sensitivity and 70% specificity for a triage test.
Conclusion: We found that majority of the biomarkers for diagnosis of TB in children are promising but will need further refining and optimization to improve their performances. As new data are emerging, stronger emphasis should be placed on improving the design, quality and general reporting of future studies investigating TB biomarkers in children.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Beyers N. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. Int J Tuberc Lung Dis. 2006;10(3):259–63. Epub 2006/03/28. . - PubMed
-
- Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis. 2004;8(5):636–47. Epub 2004/05/13. . - PubMed
-
- Tahmeed Ahmed FS, Shamsir Ahmed A.M. Childhood Tuberculosis: A review of Epidemiology, Diagnosis and Management. Inf Dis J of Pakistan. 2008;17:52–60.
Publication types
MeSH terms
Substances
Grants and funding
- MC_EX_MR/K011944/1/MRC_/Medical Research Council/United Kingdom
- MC_EX_MR/P024270/1/MRC_/Medical Research Council/United Kingdom
- MC_UP_A900_1122/MRC_/Medical Research Council/United Kingdom
- MR/K011944/1/MRC_/Medical Research Council/United Kingdom
- SRF-2009-02-07/DH_/Department of Health/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

