A Potential Therapy Using Engineered Stem Cells Prevented Malignant Melanoma in Cellular and Xenograft Mouse Models
- PMID: 30213181
- PMCID: PMC6473263
- DOI: 10.4143/crt.2018.364
A Potential Therapy Using Engineered Stem Cells Prevented Malignant Melanoma in Cellular and Xenograft Mouse Models
Abstract
Purpose: In the present study, human neural stem cells (hNSCs) with tumor-tropic behavior were used as drug delivery vehicle to selectively target melanoma. A hNSC line (HB1.F3) was transduced into two types: one expressed only the cytosine deaminase (CD) gene (HB1.F3. CD) and the other expressed both CD and human interferon-β (IFN-β) genes (HB1.F3.CD. IFN-β).
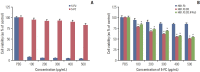
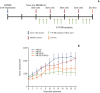
Materials and methods: This study verified the tumor-tropic migratory competence of engineered hNSCs on melanoma (A375SM) using a modified Boyden chamber assay in vitro and CM-DiI staining in vivo. The antitumor effect of HB1.F3.CD and HB1.F3.CD.IFN-β on melanoma was also confirmed using an MTT assay in vitro and xenograft mouse models.
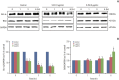
Results: A secreted form of IFN-β from the HB1.F3.CD.IFN-β cells modified the epithelial-mesenchymal transition (EMT) process and metastasis of melanoma. 5-Fluorouracil treatment also accelerated the expression of the pro-apoptotic protein BAX and decelerated the expression of the anti-apoptotic protein Bcl-xL on melanoma cell line.
Conclusion: Our results illustrate that engineered hNSCs prevented malignant melanoma cells from proliferating in the presence of the prodrug, and the form that secreted IFN-β intervened in the EMT process and melanoma metastasis. Hence, neural stem cell-directed enzyme/prodrug therapy is a plausible treatment for malignant melanoma.
Keywords: Cytosine deaminase; Flucytosine; Human interferon-β; Human neural stem cells; Melanoma.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures




Similar articles
-
Anti-proliferative Effect of Engineered Neural Stem Cells Expressing Cytosine Deaminase and Interferon-β against Lymph Node-Derived Metastatic Colorectal Adenocarcinoma in Cellular and Xenograft Mouse Models.Cancer Res Treat. 2017 Jan;49(1):79-91. doi: 10.4143/crt.2015.503. Epub 2016 May 3. Cancer Res Treat. 2017. PMID: 27188205 Free PMC article.
-
Selective antitumor effect of neural stem cells expressing cytosine deaminase and interferon-beta against ductal breast cancer cells in cellular and xenograft models.Stem Cell Res. 2014 Jan;12(1):36-48. doi: 10.1016/j.scr.2013.09.010. Epub 2013 Oct 1. Stem Cell Res. 2014. PMID: 24141111
-
Suppression of the growth of human colorectal cancer cells by therapeutic stem cells expressing cytosine deaminase and interferon-β via their tumor-tropic effect in cellular and xenograft mouse models.Mol Oncol. 2013 Jun;7(3):543-54. doi: 10.1016/j.molonc.2013.01.004. Epub 2013 Jan 19. Mol Oncol. 2013. PMID: 23403306 Free PMC article.
-
Antithyroid cancer effects of human neural stem cells expressing therapeutic genes on anaplastic thyroid cancer cells.J Cell Biochem. 2020 Feb;121(2):1586-1598. doi: 10.1002/jcb.29393. Epub 2019 Sep 12. J Cell Biochem. 2020. PMID: 31512776
-
Synergistic effects of genetically engineered stem cells expressing cytosine deaminase and interferon-β via their tumor tropism to selectively target human hepatocarcinoma cells.Cancer Gene Ther. 2012 Sep;19(9):644-51. doi: 10.1038/cgt.2012.45. Epub 2012 Jul 13. Cancer Gene Ther. 2012. PMID: 22790964
Cited by
-
Next-generation stem cells - ushering in a new era of cell-based therapies.Nat Rev Drug Discov. 2020 Jul;19(7):463-479. doi: 10.1038/s41573-020-0064-x. Epub 2020 Apr 6. Nat Rev Drug Discov. 2020. PMID: 32612263 Review.
-
Treating Metastatic Brain Cancers With Stem Cells.Front Mol Neurosci. 2021 Nov 24;14:749716. doi: 10.3389/fnmol.2021.749716. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34899179 Free PMC article. Review.
-
Construction of tetravalent bispecific Tandab (CD3/BCMA)-secreting human umbilical cord mesenchymal stem cells and its efficiency in the treatment of multiple myeloma.Stem Cell Res Ther. 2025 Feb 12;16(1):69. doi: 10.1186/s13287-025-04212-w. Stem Cell Res Ther. 2025. PMID: 39939863 Free PMC article.
-
A facile and scalable in production non-viral gene engineered mesenchymal stem cells for effective suppression of temozolomide-resistant (TMZR) glioblastoma growth.Stem Cell Res Ther. 2020 Sep 11;11(1):391. doi: 10.1186/s13287-020-01899-x. Stem Cell Res Ther. 2020. PMID: 32917269 Free PMC article.
-
Practical Use of Immortalized Cells in Medicine: Current Advances and Future Perspectives.Int J Mol Sci. 2023 Aug 12;24(16):12716. doi: 10.3390/ijms241612716. Int J Mol Sci. 2023. PMID: 37628897 Free PMC article. Review.
References
-
- American Cancer Society . Cancer facts and figures 2017. Atlanta, GA: American Cancer Society; 2017.
-
- Berwick M, Buller DB, Cust A, Gallagher R, Lee TK, Meyskens F, et al. Melanoma epidemiology and prevention. Cancer Treat Res. 2016;167:17–49. - PubMed
-
- Russak JE, Rigel DS. Risk factors for the development of primary cutaneous melanoma. Dermatol Clin. 2012;30:363–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

