Comparing Asthma Control Questionnaire (ACQ) and National Asthma Education and Prevention Program (NAEPP) asthma control criteria
- PMID: 30213611
- PMCID: PMC6309658
- DOI: 10.1016/j.anai.2018.09.448
Comparing Asthma Control Questionnaire (ACQ) and National Asthma Education and Prevention Program (NAEPP) asthma control criteria
Abstract
Background: Adequate assessment of control is critical to asthma management. The Asthma Control Questionnaire (ACQ) and the National Asthma Education and Prevention Program (NAEPP) criteria are commonly used measures of asthma control.
Objective: To examine the associations between the ACQ and NAEPP criteria and compare the validity in association with lung function, asthma exacerbation, and quality of life.
Methods: The ACQ and the NAEPP criteria were administered to 373 adolescents with asthma aged 12 to 20 years. The 2 measures correlated with forced expiratory volume in 1 second (FEV1), asthma exacerbation (oral corticosteroid use, hospitalization, and emergency department [ED] use) in the past 12 months, and quality of life.
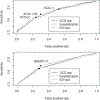
Results: Agreement between the ACQ and NAEPP criteria was moderate (κ = 0.40-0.61). Neither of the 2 measures was a reliable predictor of FEV1 less than 80% because of the high rate of false-positive results for the ACQ (68%) and low sensitivity for the NAEPP (49%). The NAEPP identified more cases of uncontrolled asthma (84.6%) than the ACQ (64.6%). The ACQ was a significant predictor of recent oral corticosteroid use, hospitalization, and ED visits (area under the curve = 0.66, 0.66, and 0.64, respectively; P < .001), as was NAEPP (area under the curve = 0.63, 0.66, and 0.61, respectively; P < .001). Both measures were significantly associated with quality of life, and the associations were particularly strong for the ACQ (r = -0.87 for symptom subscale, r = -0.76 for activity subscale, and r = -0.78 for emotional function subscale).
Conclusion: Neither the ACQ nor the NAEPP appears to reliably predict lung function, whereas both measures reasonably associate with acute asthma exacerbation. The ACQ may be the superior measure in gauging the psychosocial effect of asthma control given its particularly strong associations with quality of life.
Trial registration: ClinicalTrials.gov Identifier: NCT02293499.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest: none
Figures



References
-
- Sullivan PW, Ghushchyan V, Navaratnam P, et al. National Prevalence of Poor Asthma Control and Associated Outcomes Among School-Aged Children in the United States. J Allergy Clin Immunol Pract 2018;6(2):536–544.e1. doi: S2213-2198(17)30525-1 [pii]. - PubMed
-
- Strunk RC, Weiss ST, Yates KP, et al. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol 2006;118(5):1040–1047. doi: S0091-6749(06)01715-5 [pii]. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

