Inhibitors and chemical probes for molecular chaperone networks
- PMID: 30213856
- PMCID: PMC6369302
- DOI: 10.1074/jbc.TM118.002813
Inhibitors and chemical probes for molecular chaperone networks
Abstract
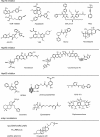
The molecular chaperones are central mediators of protein homeostasis. In that role, they engage in widespread protein-protein interactions (PPIs) with each other and with their "client" proteins. Together, these PPIs form the backbone of a network that ensures proper vigilance over the processes of protein folding, trafficking, quality control, and degradation. The core chaperones, such as the heat shock proteins Hsp60, Hsp70, and Hsp90, are widely expressed in most tissues, yet there is growing evidence that the PPIs among them may be re-wired in disease conditions. This possibility suggests that these PPIs, and perhaps not the individual chaperones themselves, could be compelling drug targets. Indeed, recent efforts have yielded small molecules that inhibit (or promote) a subset of inter-chaperone PPIs. These chemical probes are being used to study chaperone networks in a range of models, and the successes with these approaches have inspired a community-wide objective to produce inhibitors for a broader set of targets. In this Review, we discuss progress toward that goal and point out some of the challenges ahead.
Keywords: 70 kilodalton heat shock protein (Hsp70); chemical biology; drug discovery; heat shock protein 90 (Hsp90); high-throughput screening; inhibitor; molecular chaperone; protein folding; protein–protein interaction; proteostasis; small molecule inhibitor.
© 2019 Gestwicki and Shao.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

