Sex Differences in the Pharmacokinetics of Low-dose Ketamine in Plasma and Brain of Male and Female Rats
- PMID: 30213876
- PMCID: PMC6226548
- DOI: 10.1124/jpet.118.251652
Sex Differences in the Pharmacokinetics of Low-dose Ketamine in Plasma and Brain of Male and Female Rats
Abstract
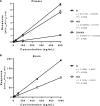
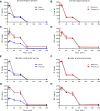
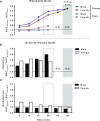
Recent work from our group and others has revealed a higher sensitivity of female rodents to the antidepressant-like effects of the N-methyl d-aspartate receptor antagonist ketamine strongly influenced by circulating estrogen and progesterone levels. However, in the absence of any preclinical studies of pharmacokinetic sex differences using low-dose ketamine in rats, it is unclear whether the effects of sex and hormonal milieu on ketamine's behavioral actions are influenced by differences in ketamine metabolism between male and female rats. Therefore, this work examined whether sex and hormonal status affect ketamine metabolism and distribution in male and female rats using a low antidepressant-like dose selectively effective in females. Intact male rats and female rats in either diestrus (low estrogen, progesterone) or proestrus (high estrogen, progesterone) were administered low-dose ketamine, and their plasma and brains were collected to analyze levels of ketamine and its metabolites norketamine (NK) and dehydronorketamine. Females exhibited greater concentrations of ketamine and NK over the first 30 min following treatment in both brain and plasma, largely accounted for by slower clearance rates and longer half-lives. Interestingly, despite the impact of ovarian hormones on behavioral sensitivity to ketamine, no appreciable differences in pharmacokinetic parameters existed between proestrus and diestrus female rats. This work is the first to demonstrate sex differences in ketamine pharmacokinetics in rats, and suggests that while sex differences in metabolism may influence the amount of ketamine and NK reaching target areas in the brain, the impact of circulating hormone levels here is negligible.
Copyright © 2018 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, et al. (2005) Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146:1650–1673. - PubMed
-
- Carrier N, Kabbaj M. (2013) Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70:27–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

