Functional differences of short and long isoforms of spastin harboring missense mutation
- PMID: 30213879
- PMCID: PMC6177001
- DOI: 10.1242/dmm.033704
Functional differences of short and long isoforms of spastin harboring missense mutation
Abstract
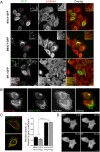
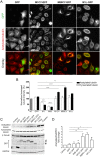
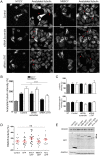
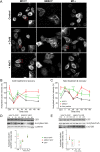
Mutations of the SPG4 (SPAST) gene encoding for spastin protein are the main causes of hereditary spastic paraplegia. Spastin binds to microtubules and severs them through the enzymatic activity of its AAA domain. Several missense mutations located in this domain lead to stable, nonsevering spastins that decorate a subset of microtubules, suggesting a possible negative gain-of-function mechanism for these mutants. Of the two main isoforms of spastin, only mutations of the long isoform, M1, are supposed to be involved in the onset of the pathology, leaving the role of the ubiquitously expressed shorter one, M87, not fully investigated and understood. Here, we show that two isoforms of spastin harboring the same missense mutation bind and bundle different subsets of microtubules in HeLa cells, and likely stabilize them by increasing the level of acetylated tubulin. However, only mutated M1 has the ability to interact with wild-type M1, and decorates a subset of perinuclear microtubules associated with the endoplasmic reticulum that display higher resistance to microtubule depolymerization and increased intracellular ionic strength, compared with those decorated by mutated M87. We further show that only mutated M1 decorates microtubules of proximal axons and dendrites, and strongly impairs axonal transport in cortical neurons through a mechanism likely independent of the microtubule-severing activity of this protein.
Keywords: HSP; Microtubules; Missense mutations; SPG4; Spastin.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
-
- Ahmad F. J. and Baas P. W. (1995). Microtubules released from the neuronal centrosome are transported into the axon. J. Cell Sci. 108, 2761-2769. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

