Copper-catalyzed methylative difunctionalization of alkenes
- PMID: 30213939
- PMCID: PMC6137206
- DOI: 10.1038/s41467-018-06246-6
Copper-catalyzed methylative difunctionalization of alkenes
Abstract
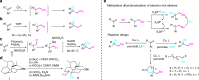
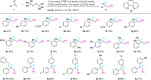
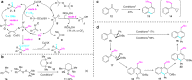
Trifluoromethylative difunctionalization and hydrofunctionalization of unactivated alkenes have been developed into powerful synthetic methodologies. On the other hand, methylative difunctionalization of olefins remains an unexplored research field. We report in this paper the Cu-catalyzed alkoxy methylation, azido methylation of alkenes using dicumyl peroxide (DCP), and di-tert-butyl peroxide (DTBP) as methyl sources. Using functionalized alkenes bearing a tethered nucleophile (alcohol, carboxylic acid, and sulfonamide), methylative cycloetherification, lactonization, and cycloamination processes are subsequently developed for the construction of important heterocycles such as 2,2-disubstituted tetrahydrofurans, tetrahydropyrans, γ-lactones, and pyrrolidines with concurrent generation of a quaternary carbon center. The results of control experiments suggest that the 1,2-alkoxy methylation of alkenes goes through a radical-cation crossover mechanism, whereas the 1,2-azido methylation proceeds via a radical addition and Cu-mediated azide transfer process.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Yan G, Borah AJ, Wang L, Yang M. Recent advances in transition metal-catalyzed methylation reactions. Adv. Synth. Catal. 2015;357:1333–1350. doi: 10.1002/adsc.201400984. - DOI
-
- Hu L, Liu YA, Liao X. Recent progress in methylation of (hetero)arenes by cross-coupling or C–H activation. Synlett. 2018;29:375–382. doi: 10.1055/s-0037-1609093. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

