High-Reynolds Microfluidic Sorting of Large Yeast Populations
- PMID: 30214051
- PMCID: PMC6137188
- DOI: 10.1038/s41598-018-31726-6
High-Reynolds Microfluidic Sorting of Large Yeast Populations
Abstract
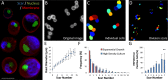
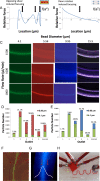
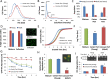
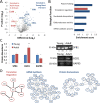
Microfluidic sorting offers a unique ability to isolate large numbers of cells for bulk proteomic or metabolomics studies but is currently limited by low throughput and persistent clogging at low flow rates. Recently we uncovered the physical principles governing the inertial focusing of particles in high-Reynolds numbers. Here, we superimpose high Reynolds inertial focusing on Dean vortices, to rapidly isolate large quantities of young and adult yeast from mixed populations at a rate of 107 cells/min/channel. Using a new algorithm to rapidly quantify budding scars in isolated yeast populations and system-wide proteomic analysis, we demonstrate that protein quality control and expression of established yeast aging markers such as CalM, RPL5, and SAM1 may change after the very first replication events, rather than later in the aging process as previously thought. Our technique enables the large-scale isolation of microorganisms based on minute differences in size (±1.5 μm), a feat unmatched by other technologies.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

