Endophthalmitis rates among patients receiving intravitreal anti-VEGF injections: a USA claims analysis
- PMID: 30214147
- PMCID: PMC6124467
- DOI: 10.2147/OPTH.S169143
Endophthalmitis rates among patients receiving intravitreal anti-VEGF injections: a USA claims analysis
Abstract
Purpose: Intravitreal (IVT) injections of the anti-vascular endothelial growth factor (VEGF) agents aflibercept, bevacizumab, and ranibizumab are commonly prescribed to treat neovascular age-related macular degeneration (nAMD). Studies comparing inflammation rates in large populations of patients receiving these agents and the treatment of ocular inflammation post-IVT anti-VEGF injections are scarce. In this study, we compared rates of endophthalmitis claims (sterile and infectious) following IVT anti-VEGF injections to determine the risk factors associated with developing endophthalmitis, and examined the claims for subsequent treatment.
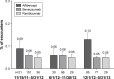
Patients and methods: This retrospective cohort study of USA claims data examined the risk of developing endophthalmitis following IVT injection of aflibercept, bevacizumab, or ranibizumab in patients with nAMD between 11/18/2011 and 5/31/2013. The primary study outcome was occurrence of endophthalmitis within 30 days of a claim for an IVT anti-VEGF injection. Endophthalmitis rates were calculated separately for aflibercept, bevacizumab, and ranibizumab, followed by pairwise comparisons of endophthalmitis frequencies among the 3 treatments.
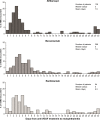
Results: This analysis included 818,558 injections from 156,594 patients with nAMD. The rates (% [n/N]) of endophthalmitis following aflibercept, bevacizumab, and ranibizumab IVT injections were 0.100% (136/135,973), 0.056% (268/481,572), and 0.047% (94/201,013), respectively. In a multivariate analysis, aflibercept was associated with a significantly higher risk of endophthalmitis vs ranibizumab (adjusted odds ratio, 2.19; 95% CI: 1.68-2.85; P<0.0001). The risk of endophthalmitis was similar for bevacizumab and ranibizumab. Within 14 days after endophthalmitis, 38.6% of cases received injectable antibiotics, 15.3% received injectable steroids, and 30.3% underwent vitrectomy.
Conclusion: The rate of endophthalmitis was very low, but higher following IVT injection with aflibercept compared with both bevacizumab and ranibizumab in patients with nAMD.
Keywords: aflibercept; bevacizumab; ranibizumab; regression analysis.
Conflict of interest statement
Disclosure Dr Kiss has served as a consultant to Alcon, Alimera, Allergan, Genentech, Inc., Optos, and Regeneron, and has received research funding from Alimera, Allergan, Genentech, Inc., Optos, and Regeneron. Dr Dugel has served as a consultant for Genentech, Inc., Novartis, and Regeneron. Dr Khanani has served as a consultant for Alimera, Allergan, Genentech, Inc., and Novartis Pharma AG and has received research funding from Genentech, Inc. Dr Broder and Dr Chang are employees of Partnership for Health Analytic Research, LLC, which was paid by Genentech, Inc. to conduct the research, and have served as consultants for Genentech, Inc. Dr Sun was an employee of Partnership for Health Analytic Research, LLC, at the time of preparation of this manuscript, is currently employed by Rancho Los Amigos National Rehabilitation Center, and has served as a consultant for Genentech, Inc. and Medscape from WebMD. Dr Turpcu is an employee of Genentech, Inc. The authors report no other conflicts of interest in this work.
Figures

References
-
- Genentech, Inc LUCENTIS® (ranibizumab injection) [package insert] 2015. [Accessed May 5, 2015]. Available from: http://www.gene.com/download/pdf/lucentis_prescribing.pdf.
-
- Regeneron, Inc EYLEA® (aflibercept injection) [package insert] 2015. [Accessed May 5, 2015]. Available from: https://www.regeneron.com/Eylea/eylea-fpi.pdf.
-
- Hahn P, Chung MM, Flynn HW, Jr, et al. Postmarketing analysis of aflibercept-related sterile intraocular inflammation. JAMA Ophthalmol. 2015;133(4):421–426. - PubMed
-
- Fileta JB, Scott IU, Flynn HW., Jr Meta-analysis of infectious endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic Surg Lasers Imaging Retina. 2014;45(2):143–149. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

