Engineering and monitoring cellular barrier models
- PMID: 30214484
- PMCID: PMC6134550
- DOI: 10.1186/s13036-018-0108-5
Engineering and monitoring cellular barrier models
Abstract
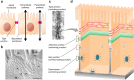
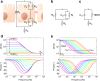
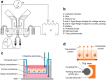
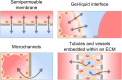
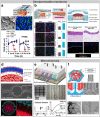
Epithelia and endothelia delineate tissue compartments and control their environments by regulating the passage of ions and solutes. This barrier function is essential for the development and maintenance of multicellular organisms, and its dysfunction is associated with numerous human diseases. Recent advances in biomaterials and microfabrication technologies have evolved in vitro approaches for modelling biological barriers. Current microphysiological systems have become more efficient and reliable in mimicking the cell microenvironment. Additionally, methods for the quantification of barrier permeability have long provided significant insight into their underlying mechanisms. In this review, we outline the current techniques to quantify the barrier function of engineered tissues, and we also give an overview of recent microphysiological systems of biological barriers that emulate the microenvironment and microarchitecture of native tissues.
Keywords: Biological barriers; Cell barrier function; Microphysiological systems; Transepithelial electrical properties.
Conflict of interest statement
Not applicable.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

