Co-culture of bone marrow stromal cells and chondrocytes in vivo for the repair of the goat condylar cartilage defects
- PMID: 30214515
- PMCID: PMC6125981
- DOI: 10.3892/etm.2018.6551
Co-culture of bone marrow stromal cells and chondrocytes in vivo for the repair of the goat condylar cartilage defects
Abstract
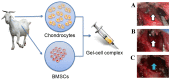
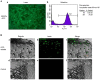
This study explored the feasibility of inducing the differentiation of BMSCs into chondrocytes through co-culture with chondrocytes in hydrogel constructs (Pluronic F-127 gel) in vivo for the repair of goat mandibular condylar cartilage defects. Chondrocytes and BMSCs were isolated from goat auricular cartilage and bone marrow, respectively, and were mixed at a ratio of 3:7. BMSCs were labelled with green fluorescence protein (GFP) using a retrovirus vector for tracing. Mixed cells were re-suspended in 30% Pluronic F-127 at a concentration of 5×107 cells/ml to form a gel-cell complex. The gel-cell complex was implanted into the temporomandibular joint condylar articular cartilage defects. The whole temporomandibular joint and adjacent tissues were harvested at 4, 8, and 12 weeks after surgery, and gross observation, histology and collagen II expression were evaluated. In the co-culture group, cartilage-like tissues were formed, and abundant type II collagen could be detected by immunohistochemistry in the condylar cartilage defects. Confocal microscopy revealed that implanted GFP-labelled BMSCs were embedded in cartilage-like tissues. The co-culture system described herein provides a chondrogenic microenvironment to induce the chondrogenic differentiation of BMSCs in vivo without any additional cellular factors.
Keywords: articular; bone marrow stromal cells; condylar; temporomandibular joint.
Figures



Similar articles
-
[Comparison study of tissue engineered cartilage constructed with chondrocytes derived from porcine auricular and articular cartilage].Zhonghua Zheng Xing Wai Ke Za Zhi. 2014 Jan;30(1):33-40. Zhonghua Zheng Xing Wai Ke Za Zhi. 2014. PMID: 24754196 Chinese.
-
[Potential of chondrogenesis of bone marrow stromal cells co-cultured with chondrocytes on biodegradable scaffold: in vivo experiment with pigs and mice].Zhonghua Yi Xue Za Zhi. 2007 Jul 17;87(27):1929-33. Zhonghua Yi Xue Za Zhi. 2007. PMID: 17923021 Chinese.
-
[Repair of articular cartilage defects by autologous bone mesenchymal stem cells and allogeneic costal chondrocytes in the knee of Wuzhishan miniature pigs].Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017 Aug 28;42(8):919-926. doi: 10.11817/j.issn.1672-7347.2017.08.008. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017. PMID: 28872083 Chinese.
-
In vitro chondrogenesis of the goat bone marrow mesenchymal stem cells directed by chondrocytes in monolayer and 3-dimetional indirect co-culture system.Chin Med J (Engl). 2011 Oct;124(19):3080-6. Chin Med J (Engl). 2011. PMID: 22040560
-
Repair of porcine articular osteochondral defects in non-weightbearing areas with autologous bone marrow stromal cells.Tissue Eng. 2006 Nov;12(11):3209-21. doi: 10.1089/ten.2006.12.3209. Tissue Eng. 2006. PMID: 17518635
Cited by
-
Therapeutic Agents for the Treatment of Temporomandibular Joint Disorders: Progress and Perspective.Front Pharmacol. 2021 Jan 29;11:596099. doi: 10.3389/fphar.2020.596099. eCollection 2020. Front Pharmacol. 2021. PMID: 33584275 Free PMC article. Review.
-
Research progress on tissue engineering in repairing tempomandibular joint.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021 Apr 25;50(2):212-221. doi: 10.3724/zdxbyxb-2021-0118. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34137227 Free PMC article. English.
-
Mesenchymal Stem Cell-Based Therapies for Temporomandibular Joint Repair: A Systematic Review of Preclinical Studies.Cells. 2024 Jun 6;13(11):990. doi: 10.3390/cells13110990. Cells. 2024. PMID: 38891122 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
