Novel strategy of sirolimus plus thymalfasin and huaier granule on tumor recurrence of hepatocellular carcinoma beyond the UCSF criteria following liver transplantation: A single center experience
- PMID: 30214575
- PMCID: PMC6126158
- DOI: 10.3892/ol.2018.9226
Novel strategy of sirolimus plus thymalfasin and huaier granule on tumor recurrence of hepatocellular carcinoma beyond the UCSF criteria following liver transplantation: A single center experience
Abstract
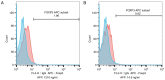
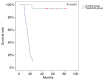
Although liver transplantation (LT) lengthens the survival time of patients with hepatocellular carcinoma (HCC), LT patients exhibit a high recurrence rate; particularly those that had advanced HCC associated with the tumor biological characteristics and long-term application of immunosuppressants. A consensus on optimal prophylaxis and treatment for recurrent HCC following LT does not currently exist. The present study retrospectively analyzed data from 36 non-University of California at San Francisco criteria-eligible patients with advanced HCC who underwent LT, and then treated them with sirolimus (SRL)-based therapy with thymalfasin and huaier granules (SRL+, n=18), or with tacrolimus-based therapy (controls; n=18). The SRL+ group had significantly longer recurrence times (P=0.008) and survival times (P<0.0001) (OS, 1-year: 100%, 3-year: 94.4%, 5-year: 77.8%; DFS, 1-year: 88.9%, 3-year: 55.6%, 5-year: 50.0%). Furthermore, compared with pre-LT values and the control group, the SRL+ group had significantly lower serum α-fetoprotein (AFP) levels (both P<0.0001) and percentage of Forkhead box P3 (FoxP3)+ Treg lymphocytes (P<0.001) during the first year. In the SRL+ group, FoxP3+/cluster of differentiation (CD)8+ Treg lymphocyte percentages decreased significantly following LT (P<0.001); however, CD8+/CD3+ T-cells significantly increased (P<0.001). Levels of serum AFP and FoxP3+ Treg cells increased when tumors relapsed, and decreased to near-normal when relapse foci were cured or stabilized. SRL+ therapy may decrease AFP and Treg levels, while increasing CD8+ T cells, indicating an associated mechanism among them. In conclusion, SRL+ therapy appears to be safe and effective in preventing HCC recurrence following LT with no significant adverse events, and warrants further investigation.
Keywords: University of California at San Francisco criteria; advanced; hepatocellular carcinoma; liver transplantation; sirolimus; tumor recurrence.
Figures
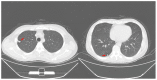
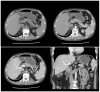
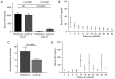
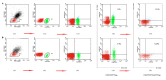
Similar articles
-
Sirolimus-based immunosuppression improves the prognosis of liver Transplantation Recipients with low TSC1/2 expression in hepatocellular carcinoma beyond the Milan Criteria.Eur J Surg Oncol. 2021 Oct;47(10):2533-2542. doi: 10.1016/j.ejso.2021.04.001. Epub 2021 Apr 19. Eur J Surg Oncol. 2021. PMID: 33902956
-
Reduction of FoxP3+ Tregs by an immunosuppressive protocol of rapamycin plus Thymalfasin and Huaier extract predicts positive survival benefits in a rat model of hepatocellular carcinoma.Ann Transl Med. 2020 Apr;8(7):472. doi: 10.21037/atm.2020.03.129. Ann Transl Med. 2020. PMID: 32395516 Free PMC article.
-
Sirolimus-based immunosuppression improves outcomes in liver transplantation recipients with hepatocellular carcinoma beyond the Hangzhou criteria.Ann Transl Med. 2020 Feb;8(4):80. doi: 10.21037/atm.2020.01.10. Ann Transl Med. 2020. PMID: 32175373 Free PMC article.
-
Meta-analysis: recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma.Aliment Pharmacol Ther. 2013 Feb;37(4):411-9. doi: 10.1111/apt.12185. Epub 2012 Dec 20. Aliment Pharmacol Ther. 2013. PMID: 23278125
-
Prophylactic liver transplantation for high-risk recurrent hepatocellular carcinoma.World J Hepatol. 2016 Nov 8;8(31):1309-1317. doi: 10.4254/wjh.v8.i31.1309. World J Hepatol. 2016. PMID: 27872682 Free PMC article. Review.
Cited by
-
LPCAT1 overexpression promotes the progression of hepatocellular carcinoma.Cancer Cell Int. 2021 Aug 21;21(1):442. doi: 10.1186/s12935-021-02130-4. Cancer Cell Int. 2021. PMID: 34419067 Free PMC article.
-
Recent Advances in the Use of Ganoderma lucidum and Coriolus versicolor Mushrooms to Enhance the Anticancer Efficacy of EGFR-Targeted Drugs in Lung Cancer.Pharmaceutics. 2025 Jul 15;17(7):917. doi: 10.3390/pharmaceutics17070917. Pharmaceutics. 2025. PMID: 40733125 Free PMC article. Review.
-
Trends of rapamycin in survival benefits of liver transplantation for hepatocellular carcinoma.World J Gastrointest Surg. 2021 Sep 27;13(9):953-966. doi: 10.4240/wjgs.v13.i9.953. World J Gastrointest Surg. 2021. PMID: 34621472 Free PMC article. Review.
-
Medicinal Mushroom Supplements in Cancer: A Systematic Review of Clinical Studies.Curr Oncol Rep. 2023 Jun;25(6):569-587. doi: 10.1007/s11912-023-01408-2. Epub 2023 Mar 30. Curr Oncol Rep. 2023. PMID: 36995535
-
PD-L1+NEUT, Foxp3+Treg, and NLR as New Prognostic Marker with Low Survival Benefits Value in Hepatocellular Carcinoma.Technol Cancer Res Treat. 2021 Jan-Dec;20:15330338211045820. doi: 10.1177/15330338211045820. Technol Cancer Res Treat. 2021. PMID: 34605709 Free PMC article. Clinical Trial.
References
-
- Yan J, Tan C, Gu F, Jiang J, Xu M, Huang X, Dai Z, Wang Z, Fan J, Zhou J. Sorafenib delays recurrence and metastasis after liver transplantation in a rat model of hepatocellular carcinoma with high expression of phosphorylated extracellular signal-regulated kinase. Liver Transpl. 2013;19:507–520. doi: 10.1002/lt.23619. - DOI - PubMed
-
- Baccarani U, Isola M, Adani GL, Benzoni E, Avellini C, Lorenzin D, Bresadola F, Uzzau A, Risaliti A, Beltrami AP, et al. Superiority of transplantation versus resection for the treatment of small hepatocellular carcinoma. Transpl Int. 2008;21:247–254. doi: 10.1111/j.1432-2277.2007.00597.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
