Oral prednisolone for acute otitis media in children: protocol of a pilot randomised, open-label, controlled study (OPAL study)
- PMID: 30214821
- PMCID: PMC6130070
- DOI: 10.1186/s40814-018-0337-x
Oral prednisolone for acute otitis media in children: protocol of a pilot randomised, open-label, controlled study (OPAL study)
Abstract
Background: Acute otitis media (AOM) is an acute inflammation of the middle ear commonly found in children, for which antibiotics are frequently prescribed. However, antibiotics are beneficial for only one third of AOM cases, and then, with only modest benefit. Since antibiotic use leads to risk of side effects and resistance, effective alternative treatments are required. Corticosteroids are a candidate because of their anti-inflammatory effects, although evidence of their efficacy and harms is insufficient. Accordingly, we plan a large, rigorous clinical trial to test this. Initially, we will test pre-specified methods and procedures (including the overall process, resources, management, and scientific components) in a pilot study of corticosteroids for AOM, which will inform a future, definitive trial.
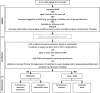
Methods: This is a pilot pragmatic, randomised, open-label, single-blind, controlled study of corticosteroids as either monotherapy or an addition to antibiotics in 60 children aged 6 months to 12 years with AOM in two cities (Jakarta and Bekasi) in Indonesia. We will randomise eligible children to prednisolone or control. We will also stratify by disease severity and randomise those with mild AOM to expectant observation plus prednisolone or observation alone and those with severe AOM to prednisolone plus antibiotic or antibiotic alone. Our outcomes are to determine (1) recruitment rates, (2) the success of the study procedures, (3) the ability to measure planned outcomes of the proposed main study, (4) the compliance to study visits and study medication, and (5) verification of the sample size calculation for the main study. We will also assess middle ear effusion using tympanometry as part of a mechanistic sub-study.
Discussion: This study will test all procedures in preparation for the main study, including several potential obstacles and challenges from the perspective of participating physicians, nurses, pharmacists, and the parents of eligible children. This information will be useful for developing strategies to overcome practical and procedural issues. This study may also provide information about the effects of corticosteroids on middle ear effusion in AOM.
Trial registration: Study registry number: ACTRN12618000049279. Name of registry: the Australian New Zealand Clinical Trials Registry (ANZCTR). Date of registration: 16 January 2018.
Keywords: Acute otitis media; Antibiotics; Corticosteroids; Mechanistic sub-study; Middle ear effusion; Trial protocol; Tympanometry.
Conflict of interest statement
This study protocol was reviewed and approved by the Ethics Committee FMUI Indonesia (No. 852/UN2.F1/ETIK/2017 and Amendment No. 1088/UN2.F1/ETIK/X/2017) and the BUHREC Australia (No. 16151 and Amendment No. 16208). We received approval for conducting clinical research from the One Stop Integrated Service Agency Province of DKI Jakarta (No. 0204/AF.1/31/-1.862.9/2017). We will also seek clinical study permits from the Training and Research division at each participating hospital. We will provide the patient information sheet, including the whole study process and procedures and potential risks from the study, and obtain consent from the parent(s) or legal guardian of patients, before conducting the recruitment and randomisation process. However, for children aged 12 years, they have also to provide their consent to participate in the study (Additional file 2. Case report forms—Pilot OPAL Study: CRF01. Information sheet and consent form). The person who delivers the consent also will provide their signatures on the consent form, stating that they have provided information and opportunity for potential participants to understand and raise relevant questions to the study. We will ensure that the consent process is free of coercion. As the participation into the study is voluntary, we will emphasise their rights to withdraw from the study at any time without any consequences, particularly on the quality of their healthcare services.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Oral prednisolone for acute otitis media in children: a pilot, pragmatic, randomised, open-label, controlled study (OPAL study).Pilot Feasibility Stud. 2020 Aug 29;6:121. doi: 10.1186/s40814-020-00671-5. eCollection 2020. Pilot Feasibility Stud. 2020. PMID: 32874679 Free PMC article.
-
A multi-centre, pragmatic, three-arm, individually randomised, non-inferiority, open trial to compare immediate orally administered, immediate topically administered or delayed orally administered antibiotics for acute otitis media with discharge in children: The Runny Ear Study (REST): study protocol.Trials. 2020 Jun 3;21(1):463. doi: 10.1186/s13063-020-04419-7. Trials. 2020. PMID: 32493407 Free PMC article.
-
Effectiveness of analgesic ear drops as add-on treatment to oral analgesics in children with acute otitis media: study protocol of the OPTIMA pragmatic randomised controlled trial.BMJ Open. 2023 Feb 22;13(2):e062071. doi: 10.1136/bmjopen-2022-062071. BMJ Open. 2023. PMID: 36813504 Free PMC article.
-
Otitis media in Greenland. Studies on historical, epidemiological, microbiological, and immunological aspects.Int J Circumpolar Health. 2001;60 Suppl 2:1-54. Int J Circumpolar Health. 2001. PMID: 11725622 Review.
-
The laws of acute otitis media.Prim Care. 2003 Mar;30(1):109-35. doi: 10.1016/s0095-4543(03)00004-6. Prim Care. 2003. PMID: 12825252 Review.
Cited by
-
Oral prednisolone for acute otitis media in children: a pilot, pragmatic, randomised, open-label, controlled study (OPAL study).Pilot Feasibility Stud. 2020 Aug 29;6:121. doi: 10.1186/s40814-020-00671-5. eCollection 2020. Pilot Feasibility Stud. 2020. PMID: 32874679 Free PMC article.
References
-
- World Health Organization: Global action plan on antimicrobial resistance. World Health Organization. 2015; http://www.who.int/iris/handle/10665/193736. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources