Profile of tildrakizumab-asmn in the treatment of moderate-to-severe plaque psoriasis: evidence to date
- PMID: 30214892
- PMCID: PMC6120577
- DOI: 10.2147/PTT.S146640
Profile of tildrakizumab-asmn in the treatment of moderate-to-severe plaque psoriasis: evidence to date
Abstract
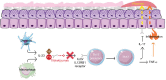
Plaque psoriasis is an immune-mediated skin disease that affects roughly 3% of adults in the United States. Advances over the past 20 years in understanding the immune-mediated pathophysiology of psoriasis have led to the development of targeted biologic therapies for this condition. Currently, biologic medications approved for the treatment of plaque psoriasis include tumor necrosis factor α inhibitors, interleukin (IL)-17 or IL-17 receptor inhibitors, IL-12/23 inhibitors, and IL-23 inhibitors. Tildrakizumab-asmn is a monoclonal antibody that targets the p19 subunit of IL-23 and is approved for use in adult patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy. This article reviews the current pharmacologic, efficacy, and safety data on tildrakizumab-asmn.
Keywords: IL-23; IL-23p19; biologics; psoriasis; tildrakizumab.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516. - PubMed
-
- Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6(6):383–392. - PubMed
-
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850. - PubMed
-
- Puig L. The role of IL 23 in the treatment of psoriasis. Expert Rev Clin Immunol. 2017;13(6):525–534. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous