Learning of spatiotemporal patterns in a spiking neural network with resistive switching synapses
- PMID: 30214936
- PMCID: PMC6135543
- DOI: 10.1126/sciadv.aat4752
Learning of spatiotemporal patterns in a spiking neural network with resistive switching synapses
Abstract
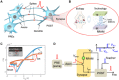
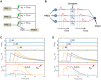
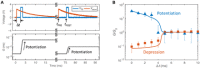
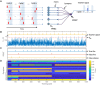
The human brain is a complex integrated spatiotemporal system, where space (which neuron fires) and time (when a neuron fires) both carry information to be processed by cognitive functions. To parallel the energy efficiency and computing functionality of the brain, methodologies operating over both the space and time domains are thus essential. Implementing spatiotemporal functions within nanoscale devices capable of synaptic plasticity would contribute a significant step toward constructing a large-scale neuromorphic system that emulates the computing and energy performances of the human brain. We present a neuromorphic approach to brain-like spatiotemporal computing using resistive switching synapses. To process the spatiotemporal spike pattern, time-coded spikes are reshaped into exponentially decaying signals that are fed to a McCulloch-Pitts neuron. Recognition of spike sequences is demonstrated after supervised training of a multiple-neuron network with resistive switching synapses. Finally, we show that, due to the sensitivity to precise spike timing, the spatiotemporal neural network is able to mimic the sound azimuth detection of the human brain.
Figures


References
-
- LeCun Y., Bengio Y., Hinton G., Deep learning. Nature 521, 436–444 (2015). - PubMed
-
- Tenenbaum J. B., Kemp C., Griffiths T. L., Goodman N. D., How to grow a mind: Statistics, structure, and abstraction. Science 331, 1279–1285 (2011). - PubMed
-
- Jun J. J., Steinmetz N. A., Siegle J. H., Denman D. J., Bauza M., Barbarits B., Lee A. K., Anastassiou C. A., Andrei A., Aydın Ç., Barbic M., Blanche T. J., Bonin V., Couto J., Dutta B., Gratiy S. L., Gutnisky D. A., Häusser M., Karsh B., Ledochowitsch P., Lopez C. M., Mitelut C., Musa S., Okun M., Pachitariu M., Putzeys J., Rich P. D., Rossant C., Sun W.-l., Svoboda K., Carandini M., Harris K. D., Koch C., O’Keefe J., Harris T. D., Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017). - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

