Clinicopathologic Features of Non-Small-Cell Lung Cancer Harboring an NTRK Gene Fusion
- PMID: 30215037
- PMCID: PMC6132056
- DOI: 10.1200/PO.18.00037
Clinicopathologic Features of Non-Small-Cell Lung Cancer Harboring an NTRK Gene Fusion
Abstract
Purpose: Gene rearrangements involving NTRK1/2/3 can generate fusion oncoproteins containing the kinase domains of TRKA/B/C, respectively. These fusions are rare in non-small cell lung cancer (NSCLC), with frequency previously estimated to be <1%. Inhibition of TRK signaling has led to dramatic responses across tumor types with NTRK fusions. Despite the potential benefit of identifying these fusions, the clinicopathologic features of NTRK fusion-positive NSCLCs are not well characterized.
Methods: We compiled a database of NSCLC cases harboring NTRK fusions. We characterized the clinical, molecular, and histologic features of these cases with central review of histology.
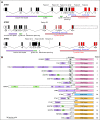
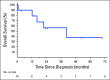
Results: We identified 11 NSCLC cases harboring NTRK gene fusions verified by next-generation sequencing (NGS) and with available clinical and pathologic data, forming the study cohort. Fusions involved NTRK1 (7 cases) and NTRK3 (4 cases), with 5 and 2 distinct fusion partners, respectively. Cohort patients were 55% male, with a median age at diagnosis of 47.6 years (range 25.3-86.0) and a median pack year history of 0 (range 0-58). 73% of patients had metastatic disease at diagnosis. No concurrent alterations in KRAS, EGFR, ALK, ROS1, or other known oncogenic drivers were identified. Nine cases were adenocarcinoma, including 2 invasive mucinous adenocarcinomas and 1 adenocarcinoma with neuroendocrine features; one was squamous cell carcinoma; and one was neuroendocrine carcinoma. By collating data on 4872 consecutively screened NSCLC cases from unique patients, we estimate a frequency of NTRK fusions in NSCLC of 0.23% (95% CI 0.11-0.40).
Conclusion: NTRK fusions occur in NSCLCs across genders, ages, smoking histories, and histologies. Given the potent clinical activity of TRK inhibitors, we advocate that all NSCLCs be screened for NTRK fusions using a multiplexed NGS-based fusion assay.
Conflict of interest statement
Relevant conflicts of interest: AFF has received consultant fees from AbbVie, PharmaMar, Loxo Oncology; research funding (to institution) from Loxo Oncology, Ignyta, AstraZeneca, AbbVie, Merck, Bristol Myers-Squibb, Novartis. RCD has received consultant/advisory board fees from Ariad, Takeda, AstraZeneca, Spectrum, and Ignyta; licensing fees from Abbott Molecular and Ignyta; stock ownership in Rain Therapeutics. VWZ has received honoraria from AstraZeneca, Roche/Genentech, Takeda, and Biocept, and consultant fees from TP Therapeutics. DLA has received consultant fees from Bristol-Myers Squibb and AbbVie LPL has equity interest and royalties from exclusive license of AMP technology to ArcherDx and has received consultant fees from ArcherDx AJI has received consulting fees for Debiopharm Group, Constellation Pharmaceuticals, Chugai Pharmaceutical, and Roche, research Funding from Blueprint Medicines, and has equity interest and royalties from exclusive license of AMP technology to ArcherDx. SHIO has received consultant/advisory board fees from ARIAD/Takeda, Pfizer, Genentech/Roche, Astra Zeneca, Novartis, Ignyta, Foundation Medicine Inc, member of the speaker bureau of Genentech/Roche, AstraZeneca, ARIAD/Takeda, and Merck; a member of the scientific advisory board of TP Therapeutics Inc and stock ownership in TP Therapeutics Inc. ATS has received consultant fees from Pfizer, Novartis, Ariad/Takeda, Genentech/Roche, Ignyta, Loxo Oncology, Blueprint medicines, KSQ therapeutics, Natera. MMK has received consultant fees from Merrimack Pharmaceuticals and H3 Biomedicine. AD has received consultant/advisory board fees from Ignyta, Loxo Oncology, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, Takeda/Ariad. MST, SK, AIS, TAB, EBH, MEA, RB, NKH, JKL have no relevant disclosures.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

