Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer
- PMID: 30215677
- PMCID: PMC6290887
- DOI: 10.1093/annonc/mdy408
Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer
Abstract
Background: A nivolumab monotherapy flat-dosing regimen of 480 mg every 4 weeks (Q4W) has been approved in several markets, including the United States, Canada, and European Union, as an alternative dosing regimen for several indications. Approvals of this Q4W regimen were based on population pharmacokinetic (PK) analyses, established flat exposure-response relationships, and clinical safety. The objective of this study was to compare the PK exposure of 480 mg Q4W with 3 mg/kg every 2 weeks (Q2W) and 240 mg Q2W using modeling and simulation, and to evaluate clinical safety of the Q4W regimen.
Patients and methods: Nivolumab PK exposure for the 480 mg Q4W schedule was simulated for 3817 patients across multiple tumor types and compared with those for the 3 mg/kg Q2W and 240 mg Q2W schedules. The safety profile of the Q4W schedule was assessed by analysis of clinical data from 61 patients who transitioned to nivolumab 480 mg Q4W from 3 mg/kg Q2W during four phase III clinical trials.
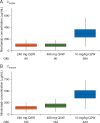
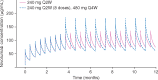
Results: Compared with 3 mg/kg Q2W, nivolumab 480 mg Q4W produced similar time-averaged concentration, approximately 16% lower trough concentration, and 45% higher peak concentration at steady state. The peak concentration for 480 mg Q4W was significantly lower than that of 10 mg/kg Q2W, a dose previously shown to have an acceptable tolerability and safety profile. Treatment-related adverse events (TRAEs) that started after transitioning from 3 mg/kg Q2W to 480 mg Q4W were reported in 14.8% of patients, with 1.6% of patients reporting grades 3-4 TRAEs. Pooled safety data for these patients are consistent with those for the 3 mg/kg Q2W schedules, and no new safety signals were identified.
Conclusions: The time-averaged steady-state exposure and safety profile of nivolumab 480 mg Q4W are consistent with that of 3 mg/kg Q2W across multiple tumor types. Nivolumab 480 mg Q4W represents a new dosing schedule option, and in addition to 240 mg Q2W, provides convenience and flexibility for patient care.
Clinical trial numbers: NCT01721772, NCT01668784, NCT01673867, NCT01642004.
Figures
Comment in
-
How do immune checkpoint-targeted antibodies work? The need for improved pharmacokinetic evaluation in early phase studies.Ann Oncol. 2018 Nov 1;29(11):2157-2160. doi: 10.1093/annonc/mdy420. Ann Oncol. 2018. PMID: 30307539 No abstract available.
References
-
- OPDIVO (nivolumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb; August 2018.
-
- OPDIVO (nivolumab) [summary of product characteristics]. Uxbridge, UK: Bristol-Myers Squibb; July 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

