Inhibitory Neuron Activity Contributions to Hemodynamic Responses and Metabolic Load Examined Using an Inhibitory Optogenetic Mouse Model
- PMID: 30215693
- PMCID: PMC6188559
- DOI: 10.1093/cercor/bhy225
Inhibitory Neuron Activity Contributions to Hemodynamic Responses and Metabolic Load Examined Using an Inhibitory Optogenetic Mouse Model
Abstract
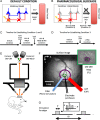
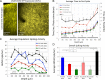
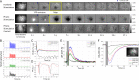
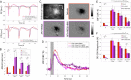
Hemodynamic signals are routinely used to noninvasively assess brain function in humans and animals. This work examined the contribution of inhibitory neuron activity on hemodynamic responses captured by changes in blood flow, volume and oxygenation in the cortex of lightly anesthetized mice. Because cortical activity is not commonly initiated by inhibitory neurons, experiments were conducted to examine the neuronal activity properties elicited by photo-stimulation. We observed comparable increases in neuronal activity evoked by forelimb and photo-stimulation; however, significantly larger increases in blood flow and volume were produced by photo-stimulation of inhibitory neurons compared with forelimb stimulation. Following blockade of glutamate and GABA-A receptors to reduce postsynaptic activity contributions, neuronal activity was reliably modulated and hemodynamic changes persisted, though slightly reduced. More importantly, photo-stimulation-evoked changes in blood flow and volume were suppressed by 75-80% with the administration of a nitric oxide synthase inhibitor, suggesting that inhibitory neurons regulate blood flow mostly via nitric oxide. Lastly, forelimb and photo-stimulation of excitatory neurons produced local decreases in blood oxygenation, while large increases were generated by photo-stimulation of inhibitory neurons. Estimates of oxygen metabolism suggest that inhibitory neuron activity has a small impact on tissue metabolic load, indicating a mismatch between the metabolic demand and blood flow regulation properties of inhibitory and excitatory neurons.
Figures



References
-
- Adachi K, Takahashi S, Melzer P, Campos KL, Nelson T, Kennedy C, Sokoloff L. 1994. Increases in local cerebral blood flow associated with somatosensory activation are not mediated by NO. Am J Physiol. 267:H2155–H2162. - PubMed
-
- Altamura C, Dell’Acqua ML, Moessner R, Murphy DL, Lesch KP, Persico AM. 2007. Altered neocortical cell density and layer thickness in serotonin transporter knockout mice: a quantitation study. Cereb Cortex. 17:1394–1401. - PubMed
-
- Armstrong-James M, Fox K, Das-Gupta A. 1992. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 68:1345–1358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

