Immunobiology of Inherited Muscular Dystrophies
- PMID: 30215857
- PMCID: PMC7769418
- DOI: 10.1002/cphy.c170052
Immunobiology of Inherited Muscular Dystrophies
Abstract
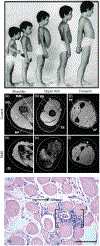
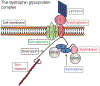
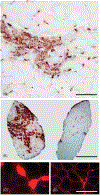
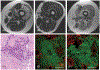
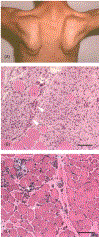
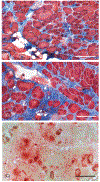
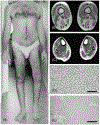
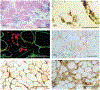
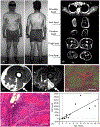
The immune response to acute muscle damage is important for normal repair. However, in chronic diseases such as many muscular dystrophies, the immune response can amplify pathology and play a major role in determining disease severity. Muscular dystrophies are inheritable diseases that vary tremendously in severity, but share the progressive loss of muscle mass and function that can be debilitating and lethal. Mutations in diverse genes cause muscular dystrophy, including genes that encode proteins that maintain membrane strength, participate in membrane repair, or are components of the extracellular matrix or the nuclear envelope. In this article, we explore the hypothesis that an important feature of many muscular dystrophies is an immune response adapted to acute, infrequent muscle damage that is misapplied in the context of chronic injury. We discuss the involvement of the immune system in the most common muscular dystrophy, Duchenne muscular dystrophy, and show that the immune system influences muscle death and fibrosis as disease progresses. We then present information on immune cell function in other muscular dystrophies and show that for many muscular dystrophies, release of cytosolic proteins into the extracellular space may provide an initial signal, leading to an immune response that is typically dominated by macrophages, neutrophils, helper T-lymphocytes, and cytotoxic T-lymphocytes. Although those features are similar in many muscular dystrophies, each muscular dystrophy shows distinguishing features in the magnitude and type of inflammatory response. These differences indicate that there are disease-specific immunomodulatory molecules that determine response to muscle cell damage caused by diverse genetic mutations. © 2018 American Physiological Society. Compr Physiol 8:1313-1356, 2018.
Copyright © 2018 American Physiological Society. All rights reserved.
Figures








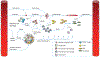



References
-
- Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sahenk Z, Weinstein M, Gardner KL, Rafael-Fortney JA, Karin M, Tidball JG, Baldwin AS, Guttridge DC. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest 117: 889–901, 2007. - PMC - PubMed
-
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17: 593–623, 1999. - PubMed
-
- Allen RE, Boxhorn LK. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J Cell Physiol 133: 567–572, 1987. - PubMed
-
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 138: 311–315, 1989. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

